B-lymphocyte, Macrophage and Mast Cell Density in the Stroma Underlying HPV-Related Cervical Squamous Epithelial Lesions and their Relationship to Disease Severity: an Immunohistochemical Study
Received: 06-Dec-2011 / Accepted Date: 28-Jan-2012 / Published Date: 01-Feb-2012 DOI: 10.4172/2161-0681.1000105
Abstract
Introduction: B lymphocyte, macrophage and mast cell densities in the stroma underlying cervical low and high grade squamous intraepithelial lesions (LSIL and HSIL) and squamous cell carcinoma (SCC) in 200 tissue samples, with concomitant HPV typing, was assessed, in order to examine their relationship to disease status and progression.
Methods: Sections from 215 cervical specimens (149 LSIL, 38 HSIL, 13 SCC and 15 normal cervical mucosae) were immunostained for B lymphocytes (CD20), macrophages (CD68) and mast cells (CD117). The number of cells per high power field (henceforth called density) in the stroma underlying epithelial lesions was assessed. Statistical analysis was performed using four ordinal scale groups of increasing severity (normal, LSIL, HSIL and SCC).
Results: Densities of all three cell types had a statistically significant, proportional correlation to disease severity, more so for B lymphocytes. Increased density of any cell type is linked to an increase in the densities of the remaining two. A statistically significant difference in B lymphocyte and mast cell density was found between LSIL and HSIL. No cell type density was found to be predictive of the outcome of LSIL. No relationship with HPV type was found.
Discussion:Increased B lymphocyte and mast cell density in the stroma underlying cervical HSIL and SCC compared to LSIL suggests a possible relationship of both cell types to progression of cervical SIL. No predictive value of the density of any cell type was found concerning the outcome of LSIL. Immunohistochemistry may contribute to elucidate the relationship of local immunity effector cells to cervical epithelial lesions.
Keywords: HPV; SIL; B-lymphocyte; Mast cell; Macrophage
308563Introduction
Since 1999, Human Papilloma Virus (HPV) infection is considered as the necessary factor in the cervical carcinogenesis process, squamous cell carcinoma (SCC) of the cervix uteri harboring HPV sequences in almost all cases. Infiltrating SCC is preceded by intraepithelial lesions, nowadays graded in a two-tiered histologic system, namely, low grade squamous intraepithelial lesion (LSIL) and high grade squamous intraepithelial lesion (HSIL), corresponding to the Bethesda classification system used in reporting cervical smears [1]. Many LSIL cases will eventually clear, probably due to effective host immunity, whereas about 15-20% will eventually progress to HSIL and about 1% to SCC [2-4]. Similarly, almost 33% of HSIL cases spontaneously regress, whereas about 12% will eventually progress to SCC [5]. These figures are indicative of the presence of host cofactors participating in the multistage HPV-related cervical carcinogenesis.
Over the past decade, evidence has accumulated concerning the pivotal role of the immune system in preventing the emergence and progression of malignancy [6-8]. On the other hand, clinical and experimental data suggest that chronic activation of the immune system during inflammatory responses may contribute to both initiation and promotion of cancer; in particular, B cell hyperactivity in the context of autoimmune or infectious diseases enhances overall cancer risk in affected tissues and organs [9, 10].
Researchers have started using mouse models of de novo or spontaneous carcinogenesis to assess the contribution of different factors of the immune system in tumor progression. Specifically, Arbeit et al. [11-13] studied the expression of the early region genes of HPV 16, controlled by the human keratin 14 promoter/enhancer in basal keratinocytes of skin and cervix. Studies based upon this model demonstrate the important role of innate and adaptive immunity in cancer development through mast cells [14] and macrophages [15], as well as the contribution of B lymphocytes through secretion of soluble factors [16], recently identified as antibodies. The latter engage receptors for the crystallizable region of IgGs (FcγRs) on the surface of immature and mature myeloid and mast cells, thus contributing to increased inflammatory infiltration, angiogenesis, epithelial hyperproliferation, and SCC development [17].
Until now, research concerning the role of the above cell types in cervical carcinogenesis has mostly been confined to mouse models. Few relevant papers concerning human tissue have up to now been published [18-22], no one dealing, to our best knowledge, with all three cell types, i.e. mast cells, macrophages and B lymphocytes. In this study, we undertook an assessment of B lymphocyte, macrophage and mast cell density in the stroma underlying LSIL, HSIL and SCC in a sample of 200 women with HPV infection, in order to try to deduce some aspects of the association between cervical squamous cell neoplasia and underlying inflammation.
Materials and Methods
Study population
The study included 187 consecutive cervical biopsy specimens from women aged 18-87, (median 36, IQR: 28-46 years) who had undergone colposcopy and cervical biopsy in the outpatient clinics of the 3rd Department of Obstetrics and Gynecology, University of Athens Medical School (“Attikon” University Hospital). According to the standard diagnostic criteria of the two-tiered SIL system [1], 149 cases were classified as LSIL and 38 cases as HSIL. In addition, 15 cases of essentially unremarkable cervical mucosa obtained from hysterectomy specimens for uterine leiomyomas (used as healthy controls), and 13 cases of infiltrating SCC were added for comparison purposes. Hence, the total study population consisted of 215 cases. All specimens were fixed in a 10% buffered formalin solution and embedded into paraffin. The initial diagnosis was re-evaluated in Hematoxylin and Eosinstained slides by two expert pathologists (P.G.F., I.G.P.). The study was approved by the “Attikon” University Hospital Institutional Review Board; all patients had given informed consent for participation in the study.
Immunohistochemistry
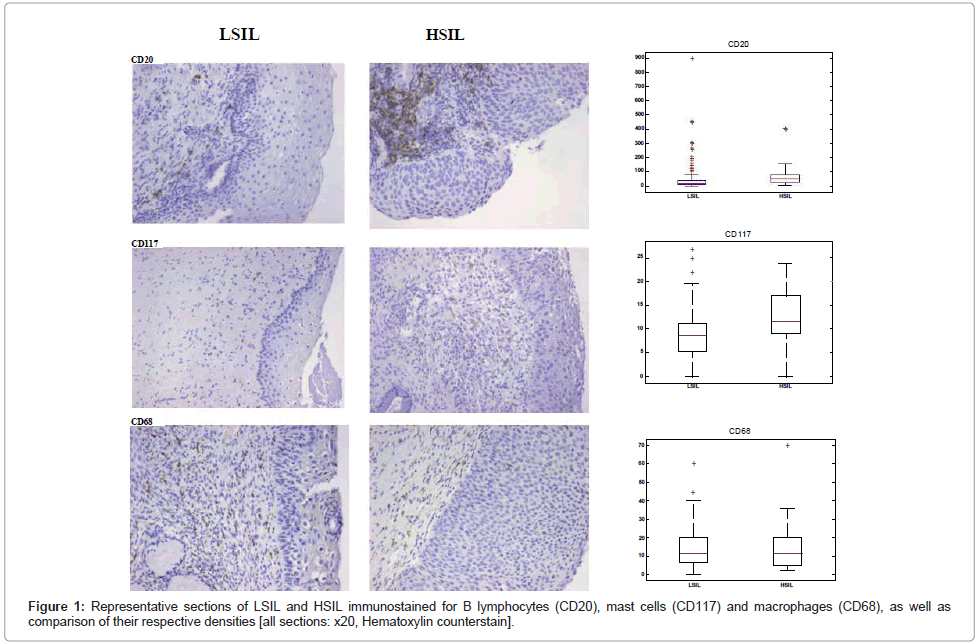
Immunostaining was performed on 3-μm deparaffinized sections; primary antibodies against CD20 (mouse monoclonal, clone L26 [DAKO], 1:300 dilution), CD117 (mouse monoclonal, clone c-kit [DAKO], 1:300 dilution) and CD68 (mouse monoclonal, clone PGM1 [DAKO], 1:50 dilution) were used. For CD20 and CD68 immunostains, slides were boiled in a microwave at 650 Watt for 20 minutes in the high pH target retrieval solution (K8004 [DAKO]) and then cooled at room temperature for 20 min; no antigen retrieval pretreatment was used for CD117 immunostaining. Endogenous peroxidase activity was blocked by means of the peroxidase-blocking reagent S2001 (K5007 [DAKO]). Immunohistochemistry was performed using the DAKO Autostainer Plus device; the antigen-antibody complex was visualized using DAB as chromogen for 10 min, following incubation with Envision/HRP for 30 min. All sections were lightly counterstained with Hematoxylin prior to mounting (Figure 1).
Scoring of immunohistochemical staining
Cell counting was done blindly using an x40 objective (=high power field, HPF). The number of immunopositive cells in the stroma underlying the epithelial lesion was assessed. Sections were assessed as follows: for B lymphocytes and macrophages the epithelial basement membrane was tangential to the uppermost part of every field, whereas for mast cells the basement membrane was always set as the horizontal diameter of every field. The reason for this difference is that previous reports have shown the highest number of mast cells to be located in the stroma immediately under the lesional epithelium [14]. For every case, two to five HPFs were examined, depending upon the extent of the lesion. The absolute number of immunostained cells within these HPFs was counted and a mean per high-power field (henceforth described as density) was calculated.
HPV genotyping
HPV genotyping was performed on DNA extracted from five, 5-μm thick sections cut from the same paraffin block used for standard Hematoxylin and Eosin staining and for immunostaining, in all 215 cases. Tissue sections were placed in a 1.5 ml sterile Eppendorf tube and DNA was extracted, amplified and analyzed using CLART® Human Papillomavirus 2 kit according to the manufacturer’s instructions (all procedures by GENOMICA, Spain) [23]. Analysis was done at the Department of Cytopathology, University of Athens Medical School, which has been accreditated by the WHO HPV Laboratory Network / HPV DNA proficiency study (both in 2009 and 2010) for use of the above mentioned kit. According to the rules of this Network, a test is proficient in typing if it can detect 50 IU / 5 ml of HPV 16 and HPV 18 DNA and 500 genome equivalents / 5 ml of the other HPV types included in the panel, both in samples with single and multiple plasmids; moreover, the specificity of the reported types should be superior to 97% (i.e. at most 1 false positive result).
Briefly, 180 μl of lysis buffer T1 and 25 μl of proteinase K were added in the aforementioned samples, which were then incubated at 56ºC for 24 h in a thermoblock with agitation. DNA extraction method was followed and 100μl of eluted DNA were produced; 5 μl of eluted DNA were used for the PCR amplification in conjunction with 1.5 μl of 25mM MgCl2. HPV DNA amplification was achieved by using biotinylated primers designed to amplify a 450bp fragment within the highly conserved L1 region of the HPV. Elimination of false negative results and PCR failure due to several PCR inhibitors inside the sample was achieved by using two internal controls: i) genomic DNA control (a pair of primers that amplify a 892bp fragment of human CFTR gene) and ii) control of the amplification reaction (a pair of primers that amplify a 1202bp fragment of modified plasmid). Hybridization of the amplified PCR product with immobilized matching type-specific DNA probes took place on a low-density microarray tube (Array Tube System, CLONDIAG Chip Technologies GmbH). Data obtained in each analysis were processed automatically by the system. CLART® HPV 2 kit allowed the detection of infection and coinfection of 35 different HPV genotypes, namely 6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 54, 56, 58, 59, 61, 62, 66, 68, 70, 71, 72, 73, 81, 82, 83, 84, 85 and 89 [24]. Diagnostic sensitivity and specificity of the CLART® HPV 2 kit amount to 98.2% and 100% respectively, according to the manufacturer.
HPV clinical arrays testing disclosed the presence of HPV infection in 168 of the 215 (78.14%) tested samples. High risk HPV types were identified in 153 of the 168 (91.07%) HPV-positive samples. Out of 15 control samples, 8 (53.33%) were HPV positive.
Statistical analysis
We used the Spearman correlation coefficient to quantify the statistical relationship between variables and to assess whether these correlations are statistically significant. The Spearman correlation lies between -1 and +1, and is interpreted as follows: the sign indicates whether the relationship is proportional (positive sign) or inversely proportional (negative sign), whereas the absolute value of the coefficient indicates how strong this relationship is. We also used the Mann-Whitney statistical hypothesis test, where the null hypothesis is that the observed distributions come from independent samples from identical continuous distributions with equal medians, against the alternative that they do not have equal medians. In all statistical tests, a p-value less than 0.05 were considered as statistically significant. We used the Matlab 2010 software package for data analysis.
Results
Two hundred and fifteen (215) cases were evaluated: 15 (7%) with unremarkable histology, 149 (69.3%) LSIL, 38 (17.7%) HSIL and 13 (6%) SCC. Single HPV type infection was identified in 58 of 168 (34.52%) HPV-positive samples, whereas the remaining 110 (65.48%) harbored multiple HPV types. Out of these 110 cases, 105 (95.45%) included at least one high risk HPV type. Concerning specific high risk HPV types, HPV16 was identified in 62 (36.9%) and HPV18 in 16 (9.52%) of the 168 HPV-positive samples. Only 15 out of 168 (8.93%) HPV-positive samples harbored low risk HPV types only.
For statistical purposes, we defined an ordinal scale 0-3 for the four examined disease groups, in increasing severity (i.e. normal, LSIL, HSIL and SCC). We then computed the Spearman correlation coefficient and the normalized mutual information (for its mathematical definition see Tsanas et al. [25]) between density of each cell type (i.e. number of CD20+, CD68+ and CD117+ cells) and the above four groups. We also reported the p-values in the Spearman test, where the null hypothesis is that there is no correlation between cell types and group, against the alternative hypothesis, i.e. that a correlation exists. The mean density of the three cell types for each group is shown in Table 1, whereas the statistical correlation by means of the Spearman test and the normalized mutual information is shown in Table 2. All three cell types appear to have statistically significant (p<0.01) correlation with disease severity, as defined in the 0-3 ordinal scale (Figure 1). In addition, we used the Mann-Whitney statistical hypothesis test to investigate whether there is a statistically significant difference in the density of each cell type between LSIL and HSIL; the results appear in the last row of Table 1, and suggest that differences in B lymphocyte and mast cell densities are statistically significant, whereas this is not the case for macrophage density (Figure 1). Practically, this suggests that there is an underlying statistical relationship correlating all three cell types and disease status. The Spearman correlation coefficient and the normalized mutual information in Table 2 aim to express the association strength of the relationships between density of each cell type and disease status. B lymphocyte density, in particular, appears to exhibit relatively stronger correlation to disease status, whereas the association strength of mast cell and macrophage density with disease status is considerably lower, though still higher than 0.2. From the sign of the Spearman correlation coefficient (positive in all three cases) we infer that the relationship between density of each cell type and the disease status is proportional; in essence, this means that the higher the density of B lymphocytes, mast cells or macrophages, the more severe is the lesion (i.e. from normal to LSIL to HSIL to SCC). Correlations between pairs of cell types are shown in Table 3; since all pairwise correlations are statistically significant with a reported correlation coefficient about 0.2, it appears that the increase in the density of any cell type is linked to an increase in the density of the remaining two cell types as well.
| B lymphocytes | Macrophages | Mast cells | Disease severity |
|---|---|---|---|
| 3.00 ± 11.13 | 10.50 ± 4.12 | 6.00 ± 2.53 | Normal histology |
| 15.50 ± 32.75 | 10.75 ± 13.75 | 8.50 ± 5.87 | LSIL |
| 47.00 ± 54.37 | 11.00 ± 15.25 | 11.50 ± 8.00 | HSIL |
| 116.00 ± 193.75 | 33.50 ± 33.75 | 10.25 ± 8.88 | SCC |
| 0.00037 | 0.707 | 0.0017 | p-values |
Values are presented as median ± interquartile range. The final row refers to the Mann-Whitney statistical hypothesis test to investigate whether there is a statistically significant difference in the cell densities between LSIL and HSIL.
Table 1: Mean density of cell types for each disease severity group
| Spearman correlations | p-values (Spearman) | Normalized mutual information | |
|---|---|---|---|
| B lymphocytes CD20 |
0.45 | p<0.001 | 0.79 |
| Macrophages CD68 |
0.21 | 0.0048 | 0.55 |
| Mast cells CD117 |
0.27 | p<0.001 | 0.32 |
The null hypothesis is that there is no correlation between each cell type and disease severity group.
Table 2: Statistical associations by means of the Spearman rank correlation test and the normalized mutual information.
| B lymphocytes | Macrophages | Mast cells | |
|---|---|---|---|
| B lymphocytes | 0.28 p<0.001 |
0.23 p=0.002 |
|
| Macrophages | 0.28 p<0.001 |
0.19 p=0.01 |
|
| Mast cells | 0.23 p=0.002 |
0.19 p=0.01 |
The null hypothesis is there is no pairwise correlation between cell types.
Table 3: Spearman correlations between pairs of cell types (including statistical significance of this correlation [p-values])
The density of B lymphocytes, mast cells and macrophages was independent of HPV subtype (using the Mann-Whitney test).
Concerning LSIL, another issue we investigated was whether the density of any particular cell type might be used as an indication of the outcome of the lesion. Accordingly, out of the total number of 149 cases of LSIL, densities of all cell types were assessed in those cases, where a follow-up Pap test (by means of liquid based cytology) was available; in those cases, cytological diagnosis was issued according to the 2001 Bethesda Classification System. The number of LSIL cases where a follow-up Pap test was available was 58; out of these, 41 (70.69%) showed regression (i.e. the follow-up Pap test was reported as negative for intraepithelial lesion or malignancy [NILM]), 13 (22.41%) remained stable (i.e. the follow-up Pap test was again reported as LSIL), whereas in the remaining 4 (6.90%), the follow-up Pap test was reported as HSIL, i.e. there was progression of the lesion. The followup Pap smear was performed 6-22 months following initial biopsy. We again used the Mann-Whitney statistical test dividing the cases in two groups: the first included the 41 cases which showed regression and the second group included both the 13 stable cases and the 4 which showed progression to HSIL (overall 17 cases). Results are summarized in Table 4 and suggest that no cell type density is predictive of the lesion outcome. Moreover, the interval between initial biopsy and the followup Pap test does not seem to affect the results shown in Table 4, again using the Mann-Whitney test.
| B lymphocytes | Macrophages | Mast cells | |
|---|---|---|---|
| p-values | 0.737 | 0.333 | 0.201 |
Table 4: Mann Whitney statistical hypothesis test to investigate whether cell densities can be used to predict of the outcome of LSIL.
Concerning the relationship of cell density to HPV status of the control samples used, we found no statistically significant difference for any of the three cell types assessed.
Concerning the relationship of HPV typing to LSIL progression, we found that in 13 (76.47%) cases of the combined stable/progressive LSIL group (including all 4 cases which showed progression to HSIL), HPV genotyping revealed infection by one or multiple high risk HPV types. More specifically, HPV16 was present in 6 (46.15%) of these cases. However, no statistically significant correlation was found between HPV type and behavior of the lesion (regression, persistence or progression) using the Mann-Whitney test (p=0.998).
To evaluate the combination of markers and their relationship to disease severity, we used a range of robust classifiers: k-Nearest Neighbours (kNN), support vector machines (SVM), Random Forests (RF) and Logistic Regression (LR). The aim is to use the markers and attempt to classify the samples as belonging to one of the four possible classes (normal, LSIL, HSIL, SCC). We have found that RF gives the lowest misclassification rate: (mean ± standard deviation) 0.240 ± 0.098. Overall then, in 76% we can detect the sample as belonging to the correct class (for details, see the confusion matrix in Table 5).
| Y=0 | Y=1 | Y=2 | Y=3 | |
|---|---|---|---|---|
| Y=0 | 114 | 37 | 0 | 0 |
| Y=1 | 24 | 1000 | 52 | 2 |
| Y=2 | 0 | 195 | 65 | 10 |
| Y=3 | 3 | 43 | 16 | 39 |
Table 5: Confusion matrix using Random forests.
Discussion
Since 1999, it has been shown that carcinoma of the cervix uteri, the second most common malignancy in women worldwide [26], is a consequence of chronic HPV infection of cervical epithelial cells [27]; this constitutes a major breakthrough in the elucidation of the etiology of cervical cancer. Among high-risk oncogenic HPV types, HPV16 followed by HPV18 are the two most common types detected by molecular methods in cervical SCC [28,29]. Infiltrating SCC is preceded by a continuum of intraepithelial lesions, collectively termed squamous intraepithelial lesions (SIL), which represent the morphologic equivalent of successive genetic and epigenetic events occurring in infected cells [30]. Nevertheless, the natural history of SIL has not yet been fully elucidated; thus, not all women infected with high-risk HPV types will eventually develop cervical carcinoma; moreover, the majority of SIL (especially low grade ones) will regress on follow-up cytological and/or histological examination [2,3]. Therefore, although features of HPV infection are implicated in the expression of a malignant phenotype by infected cells [31], host factors, especially the type and level of activation of innate and adaptive immunity, as well as the consequent inflammatory reaction in the cervix, may modulate the natural history of cervical carcinogenesis.
In this study, we have found a statistically significant correlation between increasing B lymphocyte, mast cell or macrophage density and increasing severity of the cervical epithelial lesion (LSIL, HSIL, SCC). Moreover, this correlation is statistically strong (0.45) for B lymphocytes, in line with previous reports, according to which B lymphocytes seem to behave as inhibitors of anti-tumor immunity [31-34]. One must note that the above statistical relationship may not necessarily hold for particular pairs within the defined ordinal scale, i.e. the increasing grade of severity of the lesions: thus, for example, as shown in Table 1, macrophage density does not appear to effectively differentiate LSIL from HSIL.
Our results are in accordance to those of the K14-HPV16 transgenic mouse model of multistage epithelial carcinogenesis. In this model, the early region genes of HPV16 are under control of the human keratin 14 promoter/enhancer [11]. Such mice develop epidermal hyperplasia by 1 month of age, followed by dysplasia and, finally, by development of cutaneous SCC within the first year of age in about 60% of the animals [12]. Moreover, similar epithelial pathology has been observed in the vulva and cervix of these mice [13]. K14-HPV16 transgenic mice deficient for B and T lymphocytes (K14-HPV16/RAG1-/-) show a 43% reduction in the incidence of SCC, which is related to reduced inflammation and consequently to reduced angiogenesis and epithelial proliferation; the adoptive transfer of B lymphocytes or serum from K14-HPV16 to K14-HPV16/RAG1-/- mice results in an increase of the incidence of SCC, comparable to that seen in the original strain of transgenic mice. This indicates that soluble factors secreted by B lymphocytes are important mediators of malignant progression [14]. Moreover, B lymphocyte activation in the environment of syngeneic tumors in other mice models diminishes CTL responses and drives T helper function toward Th2 polarization, thus enhancing tumorigenesis [33,35].
Although the exact contribution of B lymphocytes to each stage of human cervical carcinogenesis has not yet been fully elucidated, it has been shown that in the context of human HPV infection, B lymphocytes are recruited to sites of both premalignant [36,37] and malignant [38] cervical lesions. Moreover, antibody responses are detected in early phases of infection [39-41], asz well as in patients with cervical carcinoma [42,43]. In this regard, other types of immune cells may also be of importance.
In the aforementioned K14-HPV16 transgenic mouse model, deposition of immunoglobulins of the G isotype (IgG) in the underlying stroma of hyperplastic/dysplastic epithelium induce an inflammatory response triggered by the engagement of FcγRs on macrophages and mast cells [16,17]. Mast cells are known to be predominantly located in tissues lying at the interface between host and environment, such as skin and mucosae, where they fulfil an important role in non-allergic reactions against infectious agents through surface Toll-like receptors, FcγRs and receptors for inflammatory factors; activated mast cells communicate with other important cellular mediators of immunity against viruses, including B lymphocytes, macrophages and epithelial cells [44]. Furthermore, mast cells are increasingly recognized as critical components of the inflammatory tumor microenvironment, with most (but not all) data suggesting a negative association with prognosis in a number of human malignancies, including esophageal SCC [45], Hodgkin’s lymphoma [46] and polyoma virus-related cutaneous Merkel cell carcinoma [47]. This controversial role has also been demonstrated in in vivo studies using mouse models of multistage carcinogenesis, with both protumorigenic [48-50] and protective [51] effects reported, depending on tumor type. Regarding human cervical lesions, increased numbers of mast cells have been described in the stroma surrounding SCC compared to that underlying normal squamous epithelium [52, 53]. Moreover, a substantial increase of mast cells in the underlying stroma of cervical HSIL compared to LSIL has recently been described, allegedly related to increased microvascular density [54]. Our results appear to concur with these observations.
The absence of a significant difference in B lymphocyte and mast cell density in the underlying stroma of high risk HPV-related LSIL compared to low risk HPV-related LSIL hints at an HPV typeindependent immune involvement in lower grades of cervical SIL. Nevertheless, this has to be corroborated by larger series, since our study was performed in a small number of low risk HPV-related LSIL samples only.
Regarding macrophages, their density may be indicative of the severity of the lesion (Spearman R>0.2), although no significant differences between LSIL and HSIL were found in this study, a fact in accordance with other studies [19,20]. Moreover, in this study, we found a significant increase of macrophage density in SCC compared to HSIL, in contrast to the decrease reported in some previous studies [55,56]. This controversy might be related to the multifaceted role of tissue macrophages in immune reactions, which is related to polarization toward M1 or M2 functional phenotypes, depending on the inflammatory milieu and pathogen stimuli [57]. Apart from these, macrophages are thought to be involved in lymphangiogenesis principally in early stages of cervical carcinogenesis [21].
Despite the small number of LSIL cases with follow-up in our study, the percentages of progressing, persistent and regressing LSIL are comparable to those previously reported [3]. Concerning the predictive value of cell density for the outcome of LSIL, we found that none of the cell types assessed has such a potential, a finding in accordance to previous studies [58]; nevertheless, our results need to be corroborated using a larger sample. There are very few studies addressing the issue of lesion progression in relation to tissue immunity: in one study, regression of HSIL is related to lower numbers of stromal CD138+ plasma cells [59], whereas in another, macrophage density seems to increase in persistent or progressing LSIL in comparison to regressing ones [18]. On the other hand, assessment of systemic immunity has so far yielded controversial results: whereas one study shows that immune responses quantified by ELISPOT assays in peripheral blood do not predict regression of HSIL [60], another shows HPV16 E2- and E6- specific T lymphocyte responses (quantified by interferon-gamma ELISPOT) to correlate with progression or persistence of LSIL [61].
Despite the fact that 8 out of 15 (53.33%) cases in our control group were HPV positive, in accordance with a previous study having found a 50.7% prevalence of HPV infection in a large sample of Greek women [62], no statistically significant difference between HPV positive and HPV negative control cases was found for any of the three cell types assessed.
No statistically significant correlation was found between HPV type (as assessed by HPV genotyping) and behavior of LSIL (regression, persistence or progression) in our study. HPV genotyping has been used to assess the impact of various factors on LSIL progression in follow-up cervical smears [63]. In our study, the lack of a significant correlation might be imputed to the small sample, as previously stated.
In conclusion, our study shows an increased B lymphocyte and mast cell density in the stroma underlying cervical HSIL and SCC compared to LSIL, suggesting a possible relationship of the density of both cell types to progression of cervical SIL. Despite the fact that, in experimental mouse models, B lymphocytes have been shown to promote HPV-related carcinogenesis, whereas mast cells have a more controversial role, their specific contribution to human HPV-related cervical carcinogenesis still awaits further elucidation; in this context, immunohistochemical analysis of local immunity effector cells in cervical tissue samples may contribute to delineate their role. Moreover, we have found no correlation between the density of any of the three cell types and evolution of LSIL, as assessed by means of a follow-up Pap smear; although this might hint to some stages of HPV-related carcinogenesis being independent of specific immune effector cells, this point also needs to be further evaluated. Finally, immunohistochemical assessment of local immunity could be used in combination with other biomarkers, both in the pre-operative diagnosis and in helping to elucidate the hidden malignant potential of the disease and its eventual recurrence.
Acknowledgements
The authors wish to thank Mr K. Savvatakis, Mr C. Risilia and Mrs A. Marouga for their excellent technical support.
This work has been supported by the “Kapodistrias” research program 70/4/9109 and by the program 70/3/9326 (both of the Special Research Fund [E.L.K.E.] of the National and Kapodistrian University of Athens), as well as by the “ΑΚΑΚΟΣ” (Automated Cytological Detection of Malignancies and its Financial Effects) program (code number ATT-75), supported by the General Secretariat of Research and Technology of the Hellenic Republic.
A. Tsanas gratefully acknowledges the financial support of Intel Corporation and the Engineering and Physical Sciences Research Council (EPSRC).
References
- Wright TC, Ronnett BM, Kurman RJ, et al. (2011) Precancerous lesions of the cervix: Blaustein's Pathology of the Female Genital Tract (6thedn), Springer.
- Ostör AG (1993) Studies on 200 cases of early squamous cell carcinoma of the cervix. Int J Gynecol Pathol 12: 193-207.
- Ostör AG (1993) Natural history of cervical intraepithelial neoplasia: a critical review. Int J Gynecol Pathol 12: 186-192.
- Kyrgiou M, Valasoulis G, Founta C, Koliopoulos G, Karakitsos P, et al. (2010) Clinical management of HPV-related disease of the lower genital tract. Ann N Y Acad Sci 1205: 57-68.
- McIndoe WA, McLean MR, Jones RW, Mullins PR (1984) The invasive potential of carcinoma in situ of the cervix. Obstet Gynecol 64: 451-458.
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD (2002) Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 3: 991-998.
- Dunn GP, Old LJ, Schreiber RD (2004) The immunobiology of cancer immunosurveillance and immunoediting. Immunity 21: 137-148.
- Smyth MJ, Dunn GP, Schreiber RD (2006) Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol 90: 1-50.
- de Visser KE, Eichten A, Coussens LM (2006) Paradoxical roles of the immune system during cancer development. Nat Rev Cancer 6: 24-37.
- Grivennikov SI, Greten FR, Karin M (2010) Immunity, inflammation, and cancer. Cell 140: 883-899.
- Arbeit JM, Münger K, Howley PM, Hanahan D (1994) Progressive squamous epithelial neoplasia in K14-human papillomavirus type 16 transgenic mice. J Virol 68: 4358-4368.
- Coussens LM, Hanahan D, Arbeit JM (1996) Genetic predisposition and parameters of malignant progression in K14-HPV16 transgenic mice. Am J Pathol 149: 1899-1917.
- Arbeit JM, Howley PM, Hanahan D (1996) Chronic estrogen-induced cervical and vaginal squamous carcinogenesis in human papillomavirus type 16 transgenic mice. Proc Natl Acad Sci U S A 93: 2930-2935.
- Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, et al. (1999) Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev 13: 1382-1397.
- Giraudo E, Inoue M, Hanahan D (2004) An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Invest 114: 623-633.
- de Visser KE, Korets LV, Coussens LM (2005) De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell 7: 411-423.
- Andreu P, Johansson M, Affara NI, Pucci F, Tan T, et al. (2010) FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell 17: 121-134.
- Hammes LS, Tekmal RR, Naud P, Edelweiss MI, Kirma N, et al. (2007) Macrophages, inflammation and risk of cervical intraepithelial neoplasia (CIN) progression--clinicopathological correlation. Gynecol Oncol 105: 157-165.
- Hachisuga T, Fukuda K, Hayashi Y, Iwasaka T, Sugimori H (1989) Immunohistochemical demonstration of histiocytes in normal ectocervical epithelium and epithelial lesions of the uterine cervix. Gynecol Oncol 33: 273-278.
- al-Saleh W, Delvenne P, Arrese JE, Nikkels AF, Piérard GE, et al. (1995) Inverse modulation of intraepithelial Langerhans' cells and stromal macrophage/dendrocyte populations in human papillomavirus-associated squamous intraepithelial lesions of the cervix. Virchows Arch 427: 41-48.
- Utrera-Barillas D, Castro-Manrreza M, Castellanos E, Gutiérrez-RodrÃguez M, Arciniega-RuÃz de Esparza O, et al. (2010) The role of macrophages and mast cells in lymphangiogenesis and angiogenesis in cervical carcinogenesis. Exp Mol Pathol 89: 190-196.
- Silva CS, Michelin MA, Etchebehere RM, Adad SJ, Murta EF (2010) Local lymphocytes and nitric oxide synthase in the uterine cervical stroma of patients with grade III cervical intraepithelial neoplasia. Clinics (Sao Paulo) 65: 575-581.
- Tsiodras S, Hatzakis A, Spathis A, Margari N, Meristoudis C, et al. (2011) Molecular epidemiology of HPV infection using a clinical array methodology in 2952 women in Greece. Clin Microbiol Infect 17: 1185-1188.
- Dunne EF, Unger ER, Sternberg M, McQuillan G, Swan DC, et al. (2007) Prevalence of HPV infection among females in the United States. JAMA 297: 813-819.
- Tsanas A, Little MA, McSharry PE, Ramig LO (2011) Nonlinear speech analysis algorithms mapped to a standard metric achieve clinically useful quantification of average Parkinson's disease symptom severity. J R Soc Interface 8: 842-855.
- Parkin DM, Bray F (2006) Chapter 2: The burden of HPV-related cancers. Vaccine 24 Suppl 3: S3/11-25.
- zur Hausen H (2002) Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer 2: 342-350.
- Bosch FX, Burchell AN, Schiffman M, Giuliano AR, de Sanjose S, et al. (2008) Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine 26: 1-16.
- Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, et al. (2003) Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 348: 518-527.
- Moody CA, Laimins LA (2010) Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer 10: 550-560.
- Woodman CB, Collins SI, Young LS (2007) The natural history of cervical HPV infection: unresolved issues. Nat Rev Cancer 7: 11-22.
- Qin Z, Richter G, Schüler T, Ibe S, Cao X, et al. (1998) B cells inhibit induction of T cell-dependent tumor immunity. Nat Med 4: 627-630.
- Shah S, Divekar AA, Hilchey SP, Cho HM, Newman CL, et al. (2005) Increased rejection of primary tumors in mice lacking B cells: inhibition of anti-tumor CTL and TH1 cytokine responses by B cells. Int J Cancer 117: 574-586.
- Inoue S, Leitner WW, Golding B, Scott D (2006) Inhibitory effects of B cells on antitumor immunity. Cancer Res 66: 7741-7747.
- Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M (2010) B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature 464: 302-305.
- Syrjänen K, Väyrynen M, Castrén O, Mäntyjärvi R, Yliskoski M (1984) The relation between the type of immunoreactive cells found in human papillomavirus (HPV) lesions of the uterine cervix and the subsequent behaviour of these lesions. Arch Gynecol 234: 189-196.
- Väyrynen M, Syrjänen K, Mäntyjärvi R, Castrén O, Saarikoski S (1985) Immunophenotypes of lymphocytes in prospectively followed up human papillomavirus lesions of the cervix. Genitourin Med 61: 190-196.
- O'Brien PM, Tsirimonaki E, Coomber DW, Millan DW, Davis JA, et al. (2001) Immunoglobulin genes expressed by B-lymphocytes infiltrating cervical carcinomas show evidence of antigen-driven selection. Cancer Immunol Immunother 50: 523-532.
- Hagensee ME, Koutsky LA, Lee SK, Grubert T, Kuypers J, et al. (2000) Detection of cervical antibodies to human papillomavirus type 16 (HPV-16) capsid antigens in relation to detection of HPV-16 DNA and cervical lesions. J Infect Dis 181: 1234-1239.
- Ho GY, Studentsov Y, Hall CB, Bierman R, Beardsley L, et al. (2002) Risk factors for subsequent cervicovaginal human papillomavirus (HPV) infection and the protective role of antibodies to HPV-16 virus-like particles. J Infect Dis 186: 737-742.
- Matsumoto K, Yasugi T, Oki A, Fujii T, Nagata C, et al. (2006) IgG antibodies to HPV16, 52, 58 and 6 L1-capsids and spontaneous regression of cervical intraepithelial neoplasia. Cancer Lett 231: 309-313.
- Nguyen HH, Broker TR, Chow LT, Alvarez RD, Vu HL, et al. (2005) Immune responses to human papillomavirus in genital tract of women with cervical cancer. Gynecol Oncol 96: 452-461.
- Reuschenbach M, Waterboer T, Wallin KL, Einenkel J, Dillner J, et al. (2008) Characterization of humoral immune responses against p16, p53, HPV16 E6 and HPV16 E7 in patients with HPV-associated cancers. Int J Cancer 123: 2626-2631.
- Abraham SN, St John AL (2010) Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol 10: 440-452.
- Elpek GO, Gelen T, Aksoy NH, ErdoÄŸan A, Dertsiz L, et al. (2001) The prognostic relevance of angiogenesis and mast cells in squamous cell carcinoma of the oesophagus. J Clin Pathol 54: 940-944.
- Molin D, Edström A, Glimelius I, Glimelius B, Nilsson G, et al. (2002) Mast cell infiltration correlates with poor prognosis in Hodgkin's lymphoma. Br J Haematol 119: 122-124.
- Beer TW, Ng LB, Murray K (2008) Mast cells have prognostic value in Merkel cell carcinoma. Am J Dermatopathol 30: 27-30.
- Wedemeyer J, Galli SJ (2005) Decreased susceptibility of mast cell-deficient Kit(W)/Kit(W-v) mice to the development of 1, 2-dimethylhydrazine-induced intestinal tumors. Lab Invest 85: 388-396.
- Gounaris E, Erdman SE, Restaino C, Gurish MF, Friend DS, et al. (2007) Mast cells are an essential hematopoietic component for polyp development. Proc Natl Acad Sci U S A 104: 19977-19982.
- Soucek L, Lawlor ER, Soto D, Shchors K, Swigart LB, et al. (2007) Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat Med 13: 1211-1218.
- Sinnamon MJ, Carter KJ, Sims LP, Lafleur B, Fingleton B, et al. (2008) A protective role of mast cells in intestinal tumorigenesis. Carcinogenesis 29: 880-886.
- BenÃtez-Bribiesca L, Wong A, Utrera D, Castellanos E (2001) The role of mast cell tryptase in neoangiogenesis of premalignant and malignant lesions of the uterine cervix. J Histochem Cytochem 49: 1061-1062.
- Cabanillas-Saez A, Schalper JA, Nicovani SM, Rudolph MI (2002) Characterization of mast cells according to their content of tryptase and chymase in normal and neoplastic human uterine cervix. Int J Gynecol Cancer 12: 92-98.
- Wilk M, Liszka Ã…Â, PaleÅ„ P, Gabriel A, LaudaÅ„ski P (2010) Intensity of angiogenesis and mast cell infiltration in cervical intraepithelial and invasive lesions - are they correlated? Pathol Res Pract 206: 217-222.
- Heller DS, Hameed M, Cracchiolo B, Wiederkehr M, Scott D, et al. (2003) Presence and quantification of macrophages in squamous cell carcinoma of the cervix. Int J Gynecol Cancer 13: 67-70.
- Davidson B, Goldberg I, Gotlieb WH, Lerner-Geva L, Ben-Baruch G, et al. (1999) Macrophage infiltration and angiogenesis in cervical squamous cell carcinoma--clinicopathologic correlation. Acta Obstet Gynecol Scand 78: 240-244.
- Biswas SK, Mantovani A (2010) Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 11: 889-896.
- Syrjänen K, Väyrynen M, Castrén O, Mäntyjärvi R, Yliskoski M (1984) The relation between the type of immunoreactive cells found in human papillomavirus (HPV) lesions of the uterine cervix and the subsequent behaviour of these lesions. Arch Gynecol 234: 189-196.
- Øvestad IT, Gudlaugsson E, Skaland I, Malpica A, Kruse AJ, et al. (2010) Local immune response in the microenvironment of CIN2-3 with and without spontaneous regression. Mod Pathol 23: 1231-1240.
- Trimble CL, Peng S, Thoburn C, Kos F, Wu TC (2010) Naturally occurring systemic immune responses to HPV antigens do not predict regression of CIN2/3. Cancer Immunol Immunother 59: 799-803.
- Woo YL, van den Hende M, Sterling JC, Coleman N, Crawford RA, et al. (2010) A prospective study on the natural course of low-grade squamous intraepithelial lesions and the presence of HPV16 E2-, E6- and E7-specific T-cell responses. Int J Cancer 126: 133-141.
- Tsiodras S, Hatzakis A, Spathis A, Margari N, Meristoudis C, et al. (2011) Molecular epidemiology of HPV infection using a clinical array methodology in 2952 women in Greece. Clin Microbiol Infect 17: 1185-1188.
- Valasoulis G, Koliopoulos G, Founta C, Kyrgiou M, Tsoumpou I, et al. (2011) Alterations in human papillomavirus-related biomarkers after treatment of cervical intraepithelial neoplasia. Gynecol Oncol 121: 43-48.
Citation: Foukas PG, Zourla AP, Tsiodras S, Tsanas A, Leventakos K et al. (2012) B-lymphocyte, Macrophage and Mast Cell Density in the Stroma Underlying HPVRelated Cervical Squamous Epithelial Lesions and their Relationship to Disease Severity: an Immunohistochemical Study. J Clinic Experiment Pathol 2:105. DOI: 10.4172/2161-0681.1000105
Copyright: © 2012 Foukas PG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 15775
- [From(publication date): 3-2012 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 10921
- PDF downloads: 4854