Research Article Open Access
Bioremediation of Chromium from Fortified Solutions by Phanerochaete Chrysosporium (MTCC 787)
| Sumit Pal* and Vimala Y | |
| Department of Microbiology, GITAM institute of science, GITAM University, Visakhapatnam, Andhra Pradesh, India | |
| Corresponding Author : | Sumit Pal Research scholar, Department of Microbiology GITAM Institute of Science, GITAM University Visakhapatnam, Andhra Pradesh, India E-mail: sumitmicrobe@gmail.com |
| Received August 26, 2011; Accepted September 27, 2011; Published October 15, 2011 | |
| Citation: Pal S, Vimala Y (2011) Bioremediation of Chromium from Fortified Solutions by Phanerochaete Chrysosporium (MTCC 787). J Bioremed Biodegrad 2:127. doi:10.4172/2155-6199.1000127 | |
| Copyright: © 2011 Pal S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Pollution by chromium is of major concern as the metal is used in electroplating, metal finishing leather tanning and chromate preparation. Chromium found in water bodies and in soil is in the form of Cr (III) and Cr (VI). Cr (VI) being of particular concern because of its greater toxicity. At present various microorganisms are used for bioremediation of heavy metals from soil and water bodies. The aim of present work was to bioremediate chromium from fortified solution by a white rot fungus Phanerochaete chrysosporium (MTCC787). The potency of Phanerochaete chrysosporium was evaluated to remediate chromium from fortified solution by viable cells, microbial biosorbents and immobilized cells for the first time. The percentage removal of chromium was analyzed by UV-Visible spectrophotometer (HitachiU-2900). The study shows 99.7% Cr (VI) was removed by biosorption with Phanerochaete chrysosporium detected spectrophotometrically after 72 hrs. This study shows that Phanerochaete chrysosporium is highly potential to be used for the removal of chromium which is also a viable, eco friendly and cost effective technology for cleanup of chromium (VI).
| Keywords |
| Chromium; Phanerochaete chrysosporium (MTCC787); Viable cells; Biosorption, Immobilization |
| Introduction |
| Due to Industrialization, environmental pollution became a conundrum for human race. This industrialization introduced pollutants like lead, chromium, mercury, uranium, selenium, zinc, arsenic, cadmium, gold, silver, copper and nickel in to the environment [1]. In these heavy metals, chromium is highly toxic metal which affect human health and environment [2,3]. Chromium ions can cause epigastria pain, dermatitis, nausea, skin allergy, allergic sensitization, vomiting, lung and nervous system damage, mutagenic and carcinogenic properties, severe diarrhea and hemorrhage in human beings [4-6]. Therefore, it is necessary to remove chromium ions, especially Cr (VI), from potable water and waste water. Chromium derived from mining and refining ores, steel plants, leather tanning, aluminum productions, industrial and household sludge, iron sheet cleaning, chrome plating, fly ash from incinerators, radioactive materials, paints, alloys, inorganic chemical production, batteries, pesticides or preservatives and metal plating [7-10]. The uptake of toxic metals is by the Physico-chemical methods such as reverse osmosis, solvent extraction, lime coagulation, ion exchange, chemical precipitation, membrane separation process, oxidation-reduction, filtration, adsorption, incineration, recovery by evaporation, neutralization, electro dialysis and electrochemical treatment [11-17]. All of these methods have many drawbacks, for instance, recurring expenses with elevated capital investments, incomplete metal removal, high reagents, high energy requirements, generation of the toxic sludge or other waste products that required safe disposal [18,19] Chromium is a toxic heavy metals and because of its toxicity and various oxidation states and solution chemistry makes it a alarming topic of research. Recently, microorganisms have been reported as biological remedants and adsorbents to remove heavy metals from wastewater [20,21]. Microorganism reacts with toxic metals for chemical biotransformation which helps in bioremediation of contaminated land and oil spills [22]. Chromium occurs in the aqueous system as both trivalent and hexavalent forms , the later being of particular concern because of its greater toxicity [23,24]. The strategy of bioremediation is to detoxify chromium (VI) in to chromium (III), so that it forms a particularly insoluble Cr(OH)3 in pH range of 6-9 severely restricting its ability to migrate to soil [25]. A extensive research was done for the removal of metal ions using strains of Penicillium sp, Rhizopus sp, Aspergillus sp and Pleurotus species [26-28]. Studies have shown that treatment of Cr (VI) on the materials surfaces with the help of microorganism convert it in to non toxic and immobile Cr (III) [14]. White rot fungi i.e. Pleurotus have been used to degrade lignin a structural polymer in woody plant and this ability enables them to degrade xenobiotic pollutants [29,30]. In the same way Phanerochaete chrysosporium have lignin peroxidase, manganese peroxidase and laccase enzymes which degrade structural polymer in woody plant and this ability helpful in degradation of xenobiotic pollutants [31] and various hazardous aromatic compounds in industrial sludge and materials [32,33]. The present study attempts to evaluate the efficiency of Phanerochaete chrysosporium (MTCC 787) in different form i.e. living cell, microbial biosorbent and immobilized cell to remove chromium (VI) from fortified solutions under laboratory conditions at different physical parameters such as pH-2,4,7,9,12 and temperature of 37°C and 40°C using basal salt medium and malt extract medium . |
| Materials and Methods |
| A lyophilized culture of Phanerochaete chrysosporium (MTCC787) collected from the IMTEC Chandigarh. The organism was revived on the malt extract agar (Blakeslee's formula) and in malt extract broth under aseptic conditions as described by IMTECH Chandigarh. |
| Preparation of chromium solution |
| 282.4 mg of Potassium dichromate was added in 100 ml of distilled water and sterilized at standard condition [34]. The concentration of the Cr (VI) was 1000ppm in the stock solution. For the test solution, 0.1ml of the stock solution was added in the 0.9ml of the medium broth then the final concentration of Cr (VI) was become 100ppm in the test tube. |
| Preparation of reagent |
| 0.25 gm of 1-5 Di phenyl carbazide was added in 50ml of acetone and stored in sterilized and dried brown colored bottle [34]. |
| Bioremediation by viable cells |
| In this method the loop full culture of Phanerochaete chrysosporium taken in the test tube containing the basal salt liquid medium with dichromate solution in it at different pH 2,4,7,9,12 and another test tube as a control. The same procedure applied in case of malt extract broth also. Then these test tubes incubated at room temperature and at 40°C for 72hrs. After 72 hrs the test tubes centrifuged at 5000 rpm for 10min and then supernatant taken for the analysis by Di phenyl carbazide method for chromium and absorbance was taken at 540nm in UV-Visible spectrophotometer (Hitachi U-2900). |
| Bioremediation by microbial biosorbents |
| In this method the culture of organism was dried at 60°C for 24hrs in a hot air oven and then used for the biosorption [35]. The dried culture weigh 0.1gram and placed with the test broth i.e. basal salt and malt extract broth at different pH with control in separate test tubes and incubated at 37°C and 40°C for 72hrs and then test tubes centrifuged at 5000 rpm for 10min and then supernatant taken for the analysis by Di phenyl carbazide method for chromium at 540 nm. |
| Bioremediation by immobilised cells |
| In this method the culture treated with sodium alginate to form a slurry and then poured drop by drop in a 0.1M calcium chloride solution and then beads poured in test tubes of different pH containing broths and dichromate solution and placed at room temperature and at 40°C. After 72 hrs chromium absorbance checked at 540nm by Di phenyl carbazide method. |
| All the experiments were conducted in triplicates and verified twice. |
| Calculations |
| The percentage removal of chromium from fortified solutions was calculated. Percentage removal of chromium is equal to 100-(A/B×100) Where A is optical density of test solution (containing fungus) and B is optical density of control solution. |
| Results and Discussion |
| Bioremediation of heavy metal ions on to fungal biomass is affected by several factors; in terms of the specific surface properties of the fungal cell wall and the physico-chemical properties of the adsorption medium such as metal ion concentration, temperature and pH [36]. |
| Bioremediation/Biosorption study |
| In this study, Cr (VI) was bioremediated by the living cells, microbial biosorbent (dead cells) and immobilized cells of Phanerochaete chrysosporium MTCC787 and some selected changes has been observed. |
| Effect of pH and temprature variations |
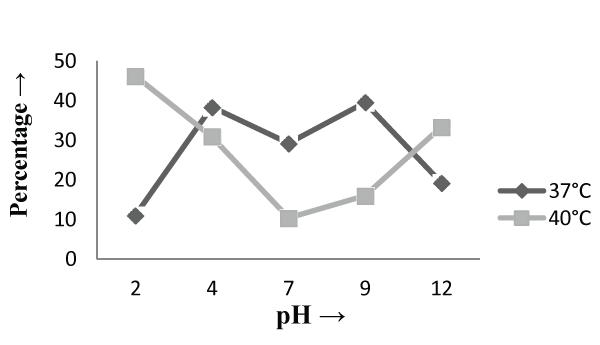
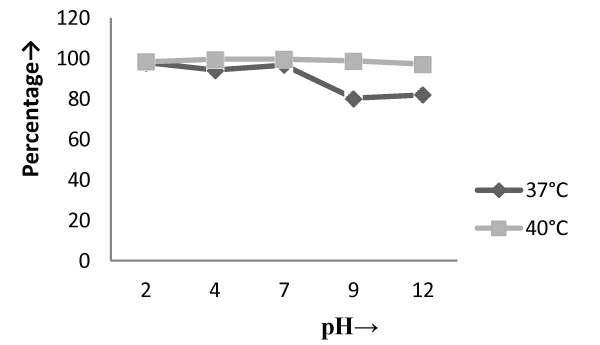
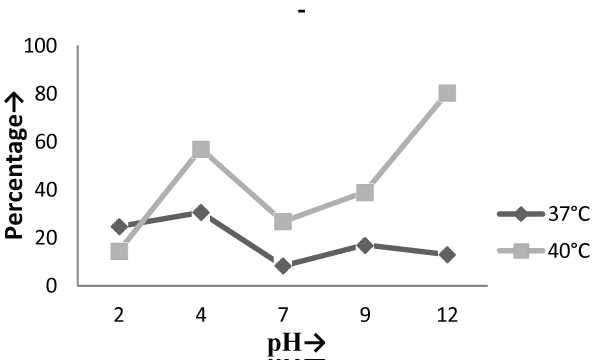
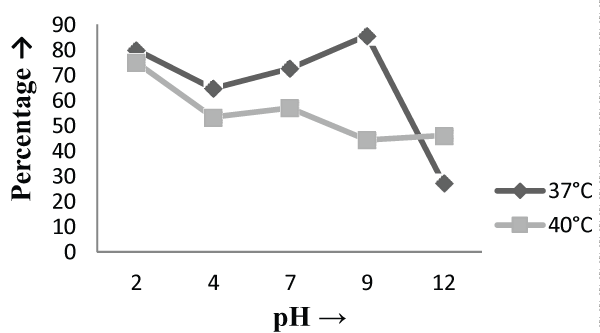
| The Experiments were conducted in order to study the effect of pH and temperature variations on the adsorption of the cellular mass. The values of pH are the most important physical parameter influencing not only site dissociation, but also the solution chemistry of the heavy metals hydrolysis, complexation by organic or inorganic legends, redox reactions, precipitation, functional groups activity in the biomass and the competition of metal ion [37] and, on the other side, strongly influence the biosorption availability of the heavy metals. The adsorption of the heavy metals like chromium depends upon the ions surrounding the fungal cell mass during the uptake of metal ions. In present study, the living cells, microbial biosorbent and immobilized cell of Phanerochaete chrysosporium MTCC787shows the difference in metal uptake capacity at different range of pH and it is due to the ion exchange, electrostatic forces, chelation, adsorption by physical forces and chemical complexation due to the pH effects. Phanerochaete chrysosporium is a crust fungus and the optimum temperature for growth for it is 40°C [38,39]. The temperature also affect the enzyme activites of manganese peroxidase and lignin peroxidase of Phanerochaete chrysosporium [40]. So the two temperature 37°C and 40°C was chosen for the study. The effect of temperature also a major cause in the biosorption of metal ions because it affect the stability of the cell wall, its configuration and can also ionized chemical moieties. These factors may simultaneously affect the binding sites on fungal species causing reduction in heavy metal removal. So the optimum temperature needed for the better removal of metals [41]. In the present study , the two different medium i.e. basal salt medium and malt extract medium were used to check the optimum medium for the Phanerochaete chrysosporium MTCC787 for the metal removal. In the basal salt liquid medium, at 37°C the viable cells shows maximum removal of chromium was 39.5% at pH 9 [Figure 1]. While the microbial biosobent and immobilized cells shows maximum percentage removal of chromium was 97.9% and 30.6% at pH 2 [Figure 3] and pH 4 [Figure 5] respectively. In earlier studies, same trend shown by marine Aspergillus niger as biosorbent, it shows 95% removal of chromium at pH 2 and at temperature 50°C [42] and it is due to the increase of hydroxide ions surrounding the adsorbent, which reduce the Cr (VI) interaction with the binding sites of cell wall of microorganism by greater repulsive forces . As the pH decreased, the overall surface on the microorganism became more active sites and adsorption increased. Thus, at acidic pH the increase of Cr (VI) biosorption show the reason by the electrostatic attraction between positively charged groups of biosorbent surface and the HCrO4- anion [43]. At 40°C the viable cells, microbial biosorbent and immobilized cells shows the maximum removal of chromium was 46.1, 99.7 and 80.3 at pH 2 [Figure 1], pH 7 [Figure 3] and pH 12 [Figure 5] respectively. Similar results were shown by Aspergillus foetidus as biosobent. A.foetidus removed 97% chromium at pH 7 [44]. In one of earlier studies Aspergillus sp N2 was able to reduce 20% Cr(VI) from solution at acidic pH while on same acidic pH Penicillium sp N3 was reduce 93% of Cr (VI) , the same study was repeated in case of neutral pH with both the species and this time Aspergillus sp. N2 reduced 74% of Cr (VI) and Penicillium sp N3 removed only 35% Cr (VI) [45]. Low removal of nickel at lower pH range by Pencillium chrysogenum has also been reported [46]. Removal of copper and lead by Micrococcus luteus [47] also showed the same trend. And the reason behind this results is that the percentage removal of chromium at high pH is depends upon the negative charge present on the fungal surface [48] due to the functional groups which helpful in the binding of cation Cr (VI) up to the optimum range of pH while at lower pH, due to the protonation of binding site resulting from high concentration of proton, negative charge intensity on the site is reduced which results in the reduction or inhibition for the binding of metal ion. |
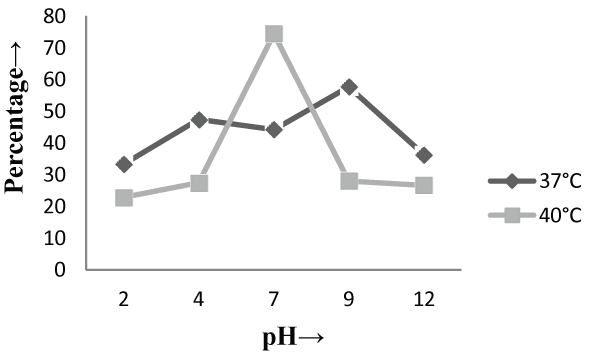
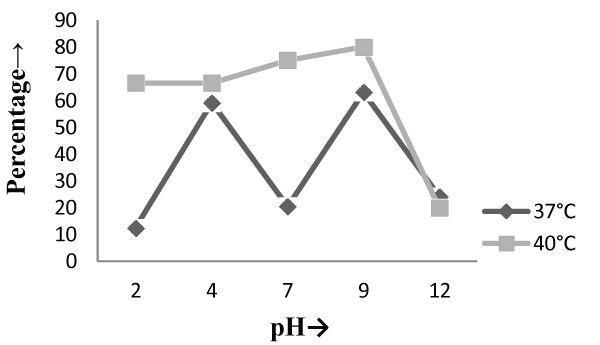
| On the other hand, in malt extract liquid medium, Phanerochaete chrysosporium MTCC787 in form of viable cells, biosorbents and immobilized cell at 37°C shows maximum chromium removal was 57.7% [Figure 2], 63.15% [Figure 4] and 85.7% [Figure 6] at pH 9 and at 40°C the viable cells, biosorbents and immobilized cells shows maximum percentage removal was 74.4%, 80 % and 75 % at pH 7 [Figure 2], pH 9 [Figure 4] and pH 2 [Figure 6] respectively. From the above findings it was proved that microbial biosorption of heavy metals from aqueous solutions depends on properties of adsorbent and molecules of adsorbate transfer from the solution to the solid phase. It has been also reported that biosorption capacities for heavy metals are strongly pH sensitive and that adsorption increases as solution pH increases [49]. The low bioaccumulation capacity at low pH is attributed to the competition of hydrogen ion with the metal ion on the sorption site. Therefore, in case of biosorption percentage removal efficiency of metal ion increases with increase in its alkalinity i.e. pH range from 2-9 is due to the relationship of bioaccumulation with the number of negative charges and which depends on dissociation of functional groups. With increase in pH beyond nine, the chromium removal rate decreased, which might be due to the osmotic changes and hydrolyzing effect [41]. Also the leaching process accelerated in the alkaline condition [50]. From the present study, Biosorption is found to be the best method with respect to living cells and immobilized cells for removal of Cr (VI) from fortified solution at pH 7 by Phanerochaete chrysosporiumMTCC787. In comparison to Gloeophyllum sepiarium, a brown rot fungus which was earlier studied for the removal of chromium from soil and that Brown rot fungus shows less then 20% removal of Cr in 1 month at pH 7.4 [51]. The bioremediation studies were conducted at the optimum temperature of 37°C and 40°C for Phanerochaete chrysosporium MTCC787. This present study shows that Phanerochaete chrysosporium MTCC787 biomass is an effective and inexpensive microbial biosorbent for chromium removal from fortified solution as compared with other fungal species. The result obtained from this study has been compared with the similar earlier study with the different fungal species [table 1]. From this study it was also clear that the Phanerochaete chrysosporium MTCC787 shows better results with basal salt medium in respect to the malt extract medium and also the optimum temperature for the Phanerochaete chrysosporium MTCC787 is 40°C for the biosorption of chromium from solution. |
| Acknowledgements |
| The authors are thankful to the Department of Microbiology, GITAM Institute of Science for providing the laboratory facilities and their constant support. |
References
|
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
 |
 |
 |
| Figure 4 | Figure 5 | Figure 6 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 15070
- [From(publication date):
November-2011 - Oct 16, 2025] - Breakdown by view type
- HTML page views : 10346
- PDF downloads : 4724
