Research Article Open Access
Biodegradation of Acetaldehyde by Microorganisms in Biological Activated Carbon Filters
| Chun-lei Z1,2*, Liang L2, Chun-bo H3 and Dong-sheng W1 | |
| 1Research Center for Eco-environmental Sciences, Chinese Academy of Sciences, Beijing 100085, China | |
| 2Water Quality Monitoring Center of Beijing Waterworks Group, Beijing 100192, China | |
| 3China University of Geosciences, Beijing 100083, China | |
| Corresponding Author : | Maulin P Shah Applied and Environmental Microbiology Lab Enviro Technology Limited (CETP) Ankleshwar-393002, Gujarat, India Fax: +91-2646-250707 E-mail: shahmp@uniphos.com |
| Received December 10, 2012; Accepted March 13, 2013; Published March 15, 2013 | |
| Citation: Shah MP, Patel KA, Nair SS, Darji AM (2013) Optimization of Environmental Parameters on Microbial Degradation of Reactive Black Dye. J Bioremed Biodeg 4:183. doi: 10.4172/2155-6199.1000184 | |
| Copyright: © 2013 Shah MP, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Biodegradation of acetaldehyde and characteristic analysis of microorganisms in Biological Activated Carbon (BAC) filter were investigated. The results revealed that microorganisms in BAC bed were mainly responsible for the removal of acetaldehyde. Biodegradation efficiency for acetaldehyde was more than 95%. The phylogenetic analysis for the nearly complete sequences of 16S rDNA demonstrated a decrease of the bacterial groups and gene sequences from 13 to 6 and 81 to 33, respectively; whilst the heteotrophic plate count bacteria increased from 1.18×107 CFU/g to 1.28×108 CFU/g. Betaproteobacteria was the predominant bacterial group, 78% of total bacteria while only 11% of those in the sample before acetaldehyde added. A lot of sequences affiliated to Betaproteobacteria, such as SY-10, SY-41, SY-1, SY-75, SY-14, SY-107 which accounted for more than 50% of total clone sequences in the clone library, had high similarity to those originated from similar environment in previous literatures and could assimilate simple organic substances containing one carbon atom. It proved they played a decisive role in the biodegradation process of acetaldehyde and were acetaldehyde degraders.
| Keywords |
| Reactive Black; Klebsiella oxytoca; Bacillus subtillis; Temperature; pH |
| Introduction |
| Rapid industrialization has necessitated the manufacture and use of different chemicals in day to day life [1,2]. The textile industry is one of them which extensively use synthetic chemicals as dyes. Wastewaters from textile industries pose a threat to the environment, as large amount of chemically different dyes are used. A significant proportion of these dyes enter the environment via wastewater [1]. Approximately 10,000 different dyes and pigments are used industrially and over 0.7 million tons of synthetic dyes are produced annually, worldwide [3]. Pollution due to textile industry effluent has increased during recent years. Moreover, it is very difficult to treat textile industry effluents because of their high Biochemical oxygen demand, Chemical oxygen demand, heat, color, pH and the presence of metal ions [4]. The textile finishing generates a large amount of waste water containing dyes and represents one of the largest causes of water pollution [5], as 10-15% of dyes are lost in the effluent during the dyeing process [6]. The traditional textile finishing industry consumes about 100 liters of water to process about 1 kg of textile material. The new closed-loop technologies such as the reuse of microbial or enzymatic treatment of dyeing effluents could help reducing this enormous water pollution [7]. Azo dyes have been used increasingly in industries because of their ease and cost effectiveness in synthesis compared to natural dyes. However, most azo dyes are toxic, carcinogenic and mutagenic [8]. Azo bonds present in these compounds are resistant to breakdown, with the potential for the persistence and accumulation in the environment [9]. Several physico-chemical techniques have been proposed for treatment of colored textile effluents. These include adsorption on different materials, oxidation and precipitation by Fenton’s reagent, bleaching with chloride or ozone photo degradation or membrane filtration [10]. All these physical or chemical methods are very expensive and result in the production of large amounts of sludge, which creates the pollution. Therefore, economic and safe removal of the polluting dyes is still an important issue. Bioremediation through microorganisms has been identified as a cost effective and environment friendly alternative for disposal of textile effluent [11,12]. In recent years a number of studies have focused on some microorganisms capable of degrading and absorbing dyes from wastewater. A wide variety of microorganisms are reported to be capable of decolonization of dyes [13-25]. |
| Materials and Methods |
| Collection of samples |
| Samples were collected from in and around Common Effluent Treatment Plant (CETP) of Ankleshwar, Gujarat, India. Samples were collected from different places, such as drainage canal that carry textile effluent to CETP, various stages of CETP. Samples were in the form of untreated effluent, treated effluent, sludge, and soil. All the samples were collected in sterile glass-screw cap tubes and preserved at 4°C in refrigerator and samples were tested within 24 hrs of collection. |
| Isolation of azo dye degrading bacteria |
| Bacterial strains were isolated through enrichment technique and Reactive Black azo dye was used as the source of carbon and nitrogen in the mineral salt medium [26]. The composition of mineral salt medium (MSM) of pH 7.2 ± 0.02 used for the isolation of bacteria was (g L-1): NaCl (1.0), CaCl2·2H2O (0.1), MgSO4·7H2O (0.5), KH2PO4 (1.0) and Na2HPO4 (1.0), yeast extract (4.0) and agar (for solid medium only) 15. Dilution plate technique was employed to obtain pure culture of bacterial strains. Bacterial strains were isolated from different wastewater samples of textile industry to use them as inocula. The 100 mg L-1 broth MSM was taken into conical flasks (250 mL), and spiked with Reactive Black (250 mg L-1) as the sole source of carbon and nitrogen. The mixture was inoculated with 5 mL of wastewater. The flasks were incubated at 32°C for 5-6 days under static conditions. After incubation, cell suspensions from each flask were plated onto MSM agar medium and incubated at 32°C for 24 h. Microbial colonies that appeared on the agar medium were re-suspended into the flasks containing fresh MSM broth (100 mg L-1) spiked with the Reactive Black dye (250 mg L-1). After an incubation of 5-6 days at 32°C, the cell suspensions were once again plated onto the agar medium. About eighty four actively growing colonies on the medium were selected from different wastewater samples. They were purified by streaking twice on agar medium. The purified cultures were preserved in refrigerator for subsequent studies. |
| Preparation of inocula |
| Liquid broth was prepared by following the MSM composition described by Khalid et al. [26]. The pH of the broth was adjusted to 7. The 100 mL of broth in 250 mL conical flask were inoculated with the respective isolated strains. Inoculated flasks were incubated under static conditions for period of 48 h at 32°C. After 48 h of incubation, optical density of 0.6 ± 0.01 was achieved at 590 nm wavelength, using optical density meter to maintain a uniform cell density. One mL of inocula was added in 10 mL medium for inoculation. |
| Screening of azo dye degrading bacteria |
| Screening was done to single out the efficient bacterial strains capable of decolorizing Reactive Black azo dye using modified MSM in a triplicate study. For this purpose, liquid MSM medium was prepared and it was spiked with 250 mg L-1 dye. The medium was inoculated with the respective inocula (84 isolates) and incubated at 32°C for 48 h. After 48 h, the medium was centrifuged at 8000 rpm for 15 min. Then decolorization was determined with the help of spectrophotometer at 597 nm by using Reactive Black dye as standard. Uninoculated media served as blanks. Percent decolorization was calculated and the five best isolates exhibiting maximum decolorization ability was selected for further experimentation. The five most effective bacterial isolates (ETL-1, ETL-2, ETL-12, ETL-14 & ETL-16) were further examined for their decolorization potentials in glass test tubes. Ten milliliters of the sterilized MSM broth containing Reactive Black dye at the concentration of 250 mg L-1 was added to the autoclaved test tubes supplemented with yeast extract (4 g L-1) as a co-substrate. The medium was inoculated with the respective bacterial strains by adding inocula of uniform cell density. The test tubes were tightly sealed to provide reduced conditions. The tubes were incubated at 32°C under static conditions. Uninoculated MSM containing azo dye in the test tubes was also incubated under similar conditions to check abiotic decolorization of the dye. The decolorization was measured at 597 nm by spectrophotometer as described by Khalid et al. [26]. |
| Optimization |
| Various factors were optimized to achieve the highest decolorization rate of Reactive Black by the selected isolates of bacteria. All the experiments were conducted in triplicate. Preliminary studies were conducted to select the standard conditions such as substrate concentration (250 mg L-1), pH (7), temperature (32°C) and static incubation. |
| Azo dye concentration |
| Five levels (50, 100, 200, 250, and 500 mg L-1) of Reactive Black azo dye were used to find the best concentration for maximum decolorization. The inoculum was added to the medium (10 mL) at inoculum: broth ratio of 1:50. Incubation temperature was 35°C. Decolorization was determined by taking 1.5 mL aliquots periodically from different test tubes and centrifuging the solutions at 8,000 rpm for 15 min to remove the cells. The absorbance of the supernatants was measured at the 597 nm, for Reactive Black by using spectrophotometer. Uninoculated blank was run to check the abiotic decolorization. Each dye level was replicated thrice. |
| Carbon source |
| In order to assess the effects of different carbon sources on bacterial decolorization, four carbon sources, namely glucose, maltose, mannitol and yeast extract were used at the rate of 4 g L-1. The MSM spiked with Reactive Black at the concentration of 250 mg L-1 was used. Incubation temperature was 35°C. Decolorization was determined by using the protocol as described above. The experiment was conducted in three replications. |
| pH |
| Effect of different pH ranging from 4 to 10 was examined on the decolorization efficiency of the selected five bacterial isolates. MSM that was enriched with Reactive Black at the concentration of 250 mg L-1 was used. Incubation temperature was maintained at 35°C. The pH was adjusted by using 0.01 M HCl or 0.01 M NaOH solutions. |
| Temperature |
| Decolorization of Reactive Black by the selected bacterial isolates was studied at different temperatures such as 25, 30, 32, 35, 40 and 42°C. Medium used for this purpose was Mineral Salt Media (MSM), which was enriched with Reactive Black at the concentration of 250 mg L-1. pH of the MSM was maintained at 7. Decolorization was determined as described above. |
| Results |
| Isolation and screening of azo dye decolorizing bacteria |
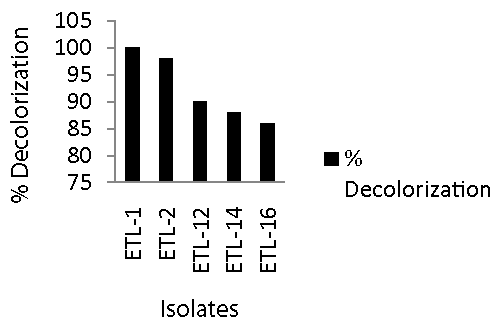
| All of the 84 bacterial isolates from the enrichment culture were screened for their efficiency to remove azo dyes from spiked broth. Bacterial isolates showed variable potential to decolorize 250 mg L-1 Reactive Black in broth medium. Only 10-15% isolates were able to decolorize the dye more than 85% in 24 h as compared to abiotic control. The majority of these isolate showed medium potential of decolorization of the dye, which ranged between 35 to 70% as compared to control. Figure 1 shows that the most efficient bacterial isolates had excellent potential to degrade Reactive Black and percent decolorization ranged from 91 to 100. The most efficient bacterial isolate in decolorizing the Reactive Black was ETL-1 that completely removed dye color. It was followed by 98% decolorization by ETL-2. Next effective strains in descending order were ETL-12 and ETL-14. The strain ETL-16 was the least efficient decolorizing strain. Based on these results, the most efficient isolates exhibiting the highest potential of decolorization in 24 h were selected for further experiments. The selected isolates were ETL-1, ETL-2, ETL-12, ETL-14 and ETL-16. |
| Substrate/dye concentration |
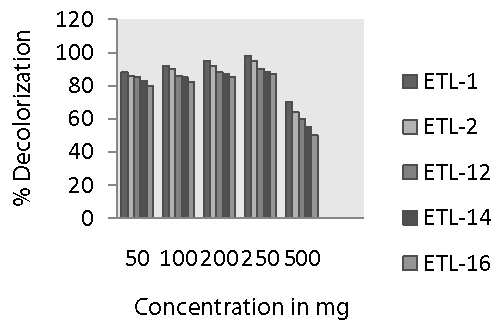
| The dye concentrations significantly affected the decolorization potential of bacteria. It is very clear from figure 2 that decolorization of Reactive Black by all the strains increased sharply when the dye concentration increased from 50 to 250 mg L-1. Maximum decolorization was observed at 250 mg L-1 of substrate concentration. Then, the rate of decolorization was reduced with the increase in dye concentration. A variation in decolorization rate by different strains of bacteria was also observed. Strain ETL-1 showed the highest decolorization at all dye concentration and complete color removal of 250 mg dye L-1 was observed after 24 h. The rate of decolorization by strain ETL-2 was almost comparable with that of ETL-1 at all tested concentration of dye. Other three strains such as ETL-12, ETL-14 and ETL-16 were relatively less efficient to remove color of the dye at different dye concentration compared to ETL-1 and ETL-2. However, like ETL-1 and ETL-2, they also showed maximum decolorization of the dye at 250 mg L-1 dye concentrations. The rate of deocorization by these three strains was also significantly lower at other dye concentrations such as 50, 100, 200 and 500 mg dye L-1. In general, strain ETL-16 was least effective to decolorize Reactive Black at all concentrations of the dye. |
| Effect of carbon and co-substrate |
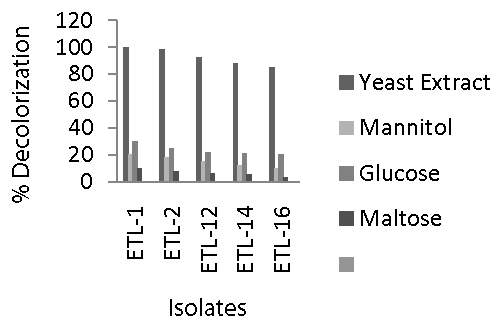
| The data regarding the effect of different carbon sources such as yeast extract, maltose, mannitol and glucose on decolorization of Reactive Black azo dye by bacterial isolates are presented in figure 3. Maximum decolorization of Reactive Black by the selected five strains of bacteria was observed when yeast extract at the rate of 4 g L-1 was applied to the liquid medium containing 250 mg L-1 dye. Decolorization ranged between 80 to 100% in 24 h. Once again, strain ETL-1 showed the most promising results and completely removed color of the dye in the presence of yeast extract in liquid medium. Next effective strain was ETL-2. However, decolorization rate by all the tested strains reduced significantly when other carbon sources such as mannitol, glucose and maltose were applied as co-substrates. The decolorization of the dye was 20 to 30% in the broth medium supplemented with glucose, while it ranged 10 to 20% when mannitol was used as a co-substrate. However, rate of decolorization by all the tested strains was lowest when maltose was supplied as a carbon source. Maximum decolorization of up to 10% was observed in the presence of maltose. In general, strain ETL- 1 performed best at all carbon sources, however significant difference with other strains was observed only in the presence of yeast extract. |
| pH |
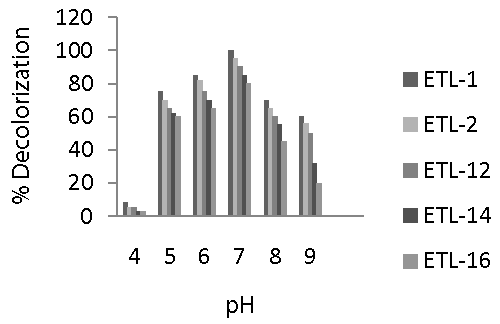
| Selected strains of bacteria were able to decolorize the dye over a wide range of pH. However, maximum decolorization by all the tested strains was recorded at pH 7 (Figure 4). Decolorization of the dye ranged from 80 to 100% at this pH. A significant increase in decolorization was observed as the pH increased from 4 to 7. However, relatively a rapid decrease in decolorization was found when pH was increased from 7 to 9. Overall, decolorization ranged from 60 to 75% at pH 5, 65 to 85% at pH 6, 80 to 100% at pH 7, 45 to 70% at pH 8 and 20 to 60% at pH 9. The strain ETL-1 was the most efficient in decolorizing Reactive Black at all levels of pH, followed by strain ETL-2. Decolorization rate of both strains was significantly different from that of ETL-12, ETL-14 and ETL-16 strains at all pH levels. In general, pH 7 favored all tested strains to decolorize the dye. |
| Temperature |
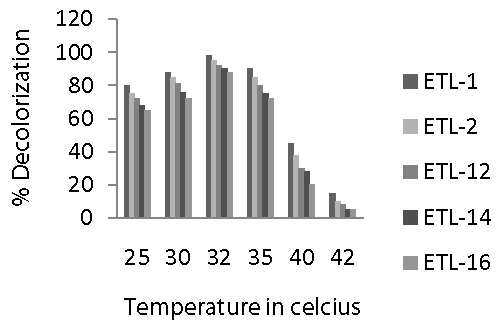
| Incubation temperature ranging from 25 to 42°C was used to find out optimal temperature for the decolorization of azo dyes by the selected strains of bacteria in liquid medium. It was observed that an increase in the temperature from 25 to 35°C had positive effect on the decolorization of Reactive Black (Figure 5). There was a significant increase in the decolorization of dye by all the strains with the increase in temperature. Optimal temperature to decolorize Reactive Black azo dye for all the tested strains was 32°C. However, decolorization rate in all strains dropped sharply as the temperature increased from 32 to 40°C. After that, there was a significant decrease in the decolorization of dye as the temperature increased from 40 to 42°C. Overall, strain ETL-1 performed best at all tested temperatures and differed markedly from other strains in terms of decolorization of azo dye. In general, a similar trend was observed in case of temperature regarding the decolorization potential of different bacterial strains as it was observed in case of other environmental factors. |
| Rate of decolorization of reactive black |
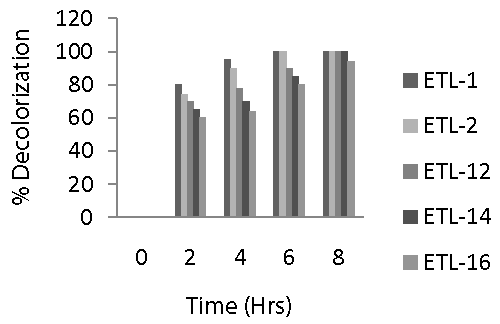
| Figure 6 revealed that bacterial strain ETL-1 was the most significant strain in decolorizing Reactive Black azo dye and caused 80, 95 and 100% decolorization in 2, 4 and 6 h, respectively. A similar trend was observed in case of strain ETL-2 that was able to completely remove 250 mg dye L-1 in 6 h under static conditions. Rate of decolorization was relatively slower in strains ETL-12, ETL-14 and ETL-16 compared to ETL-1 and ETL-2. Strain ETL-12 was able to decolorize about 90% dye in 6 h and 100% in 8 h. It was followed by the strain ETL-14 which caused 85% decolorization in 6 h, while it could decolorize 100% dye in 8 h. The strain ETL-16 was found to be the least efficient decolorizer of the dye among the tested strains. Overall, a sharp increase in the decolorization of Reactive Black by all tested strains was observed in first 2 h and then there was a gradual increase in the rate of decolorization. However, a complete removal of 100 mg dye L-1 was achieved in 6-8 h under static conditions. |
| Discussion |
| Industrial effluent is not stable and it varies often in a wide range depending upon the process practiced. South Asian countries are experiencing severe environmental problems due to rapid industrialization. This phenomenon is very common where the polluting industries like textile dyeing, leather tanning, paper and pulp processing, sugar manufacturing, etc. thrive as clusters. Among these the Textile industries are large industrial consumers of waters as well as producers of wastewater. The effluent discharged by this industry leads to serious pollution of groundwater and soils and ultimately affects the livelihood of the poor [27]. In this study, isolation of bacteria was carried out from dye-contaminated wastewater of textile industry by using Reactive Black as the sole source of carbon and nitrogen. The results demonstrated that the bacterial strains isolated through enrichment of liquid media with this dye were capable of decolorizing Reactive Black azo dye under static conditions but with highly variable potential. Out of eighty four isolates, only 2% were able to remove >80% color of the azo dye, 60% strains showed 51 to 80% decolorization while remaining 38% isolates were less efficient in decolorizing Reactive Black azo dye. Various researchers have also reported a highly variable potential of different strains of bacteria to decolorize different azo dyes [28,29]. It was found that the bacterial strain ETL-1 (Klebsiella oxytoca) was the most efficient and completely removed 100 mg L-1 of Reactive Black in 6 h. Both the strains were identified based on biochemical characterization. This may imply that certain bacteria can be effectively used for the removal of Reactive Black from textile industrial effluent. Other researchers have also reported the high potential of some bacteria to decolorize test azo dyes [30]. Since the incubation was carried out under the static conditions continuously, it is highly likely that azoreductases was the key enzyme, which decolorized the Reactive Black. Azoreductase is reported to be the key enzyme expressed in azodye- degrading bacteria and catalyses the reductive cleavage of the azo bond. Azoreductase activity had been identified in several species of bacteria, such as Caulobacter subvibrioides strain C7-D, Xenophilius azovorans KF46F, Pigmentiphaga kullae K24, Enterobacter agglomerans, Enterococcus faecalis, P. aeruginosa, Staphylococcus aureus, Shewanella putrefaciens, Pseudomonas luteola, Aeromonas hydrophila and P. hauseri [30-34]. It was further noted that the decolorization of Reactive Black was concentration dependent and maximum biodecolorization by all four bacterial strains was observed at 250 mg dye L-1 liquid medium when yeast extract was applied at the rate of 0.4% as a cosubstrate. Decolorization rate of all the four strains reduced when the concentration of dyes was either less or more than 250 mg L-1 liquid medium. Relatively low rate of decolorization of the dyes at the concentration less than 250 mg L-1 might be due to the fact that the enzymatic system involved in this reaction remained unsaturated at this substrate level or bacterial growth was not optimum at this level. On the other hand decrease in decolorization at high substrate concentration might be due to the toxic effect of the dye against the bacterial growth or enzymatic activity responsible for the degradation of the dye. Previous investigations have shown that the dye concentration does affect the rate of biodegradation and the optimum dye level could also vary from species to species, and in general higher color removal efficiencies have been observed at medium dye concentrations [26,29,35-37]. In this study, different carbon & nitrogen sources including yeast extract, mannitol, maltose and glucose were investigated for their impact on the decolorization of Reactive Black by the selected bacterial culture. Among these, only yeast extract supported the biodegradation reaction while mannitol, maltose and glucose suppressed the reaction of decolorization by the inocula. This might be due to enhanced enzymatic activity in the presence of yeast extract, while the other three carbon sources might have negative impact on the enzymatic system involved in the reaction. It is also very likely that addition of mannitol, maltose and glucose might have switched away inoculum from the target substrate (Reactive Black). Guo et al. [38] reported that bacterial strains grew well and completely decolorized K-2BP when yeast extract or peptone was present in the medium; however, glucose, glycerol, sucrose, lactose and starch resulted in lower rates of growth and decolorization of these dyes. Other researchers also have reported the maximum decolorization of azo dyes in presence of yeast extract by the bacteria they used [39]. The effect of glucose on decolorization and degradation of azo dyes by bacterial strains is controversial because some scientists [40] reported positive effect of glucose on the decolorization while other group of scientists [11,41] had opposite opinion. The negative effect of glucose on anoxic decolorization has been attributed either to a decrease in pH due to acid formation, or to catabolic repression [11]. It is also very likely that the positive or negative effect of glucose on the decolorization of azo dyes by microbial culture may depend on the concentration of glucose used to amend the media. Another limiting factor for microbial activities and azo dye decolorization is the pH of medium. Optimum pH varies among microbial strains and type of azo dye. Generally bacterial strains perform well in alkaline pH, while fungal strains prefer acidic pH. In our study it was noticed that an increase in pH from 4 to 8 significantly enhanced the rate of decolorization. However, decolorization rate was the highest between 7-8 pH. Beyond pH 8, a decrease in decolorization by all selected bacterial strains (ETL-1, ETL-2, ETL-12, ETL-14 & ETL-16) was observed. The pH 7 was proved to be the best pH for all selected isolates and decolorization up to 100% was observed in case of ETL-1 and ETL-2 at this pH. So from this study it could be concluded that the neutral pH supported bacterial activity to decolorize Reactive Black in liquid medium. Textile effluent has alkaline pH due to substantial presence of salts during dying process. High pH level of textile effluents is one of problems in their biological treatment. Therefore, tolerance to high pH is important to make this technology valuable. Prasad et al. [42] reported that Bacillus endophyticus VITABR13 strain can effectively decolorize azo dyes over a wide range of pH (6-9). However, optimum pH for growth and decolorization was found to be 8 because maximum decolorization (90%) was recorded at this pH. It is also likely that pH might also have affected the enzymatic activity involved in decolorization of dye, in addition to the cellular growth of inocula. Incubation temperature is also one of the most important parameters to study biotreatment of wastewater, as it determines the growth rate of inocula and has the impact on specific enzymatic activity. Decolorization of Reactive Black by the selected isolates was investigated over a temperature range of 25–42°C. Increase in temperature from 25 to 35°C caused increase in decolorization. Similarly further rise in temperature from 35°C to onward had negative effect on decolorization of Reactive Black. Decrease in decolorization at higher temperature may be due to the thermal deactivation of the enzyme responsible for decolorization. It is very likely that all selected isolates were mesophilic bacteria because they all showed better decolorization in the temperature range of 25 to 35°C. Previously, Guo et al. [38] reported optimum range of temperature of 28 to 35°C for the decolorization of dye contaminated by the inocula. The percentage of decolorization reached the highest value of 98% at 32°C. Over a range of 20–42°C, the specific decolorization rate increased as the temperature rose. The mesophilic range is traditionally used [43] since it is generally thought that maintaining high temperature would be uneconomical, while degradation within the psychrophilic range is too slow. Fortunately, a wide range of bacteria degrade organic pollutants efficiently in mesophilic range. |
| Conclusion |
| The present study reveals that the selected four cultures can be used successfully for decolorizing Reactive Black dye. The cultures exhibited maximum decolorization ability at pH between 7-8 and 32°C.Moreover, 4 g/L yeast extract was found to be optimum for decolorization. In conclusion, bacterial species can be studied further for bioremediation of dye-polluted waters including rate of degradation of azo dyes. |
| References |
|
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
 |
 |
 |
| Figure 4 | Figure 5 | Figure 6 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 14678
- [From(publication date):
March-2013 - Nov 08, 2025] - Breakdown by view type
- HTML page views : 9945
- PDF downloads : 4733
