Research Article Open Access
Bioaccumulation of Cadmium in Blue Green Algae Spirulina (Arthrospira) Indica
| Siva Kiran RR1*, Madhu GM2, Satyanarayana SV3 and Bindiya P4 | |
| 1Department of Biotechnology, MS Ramaiah Institute of Technology, Bangalore-560054, India | |
| 2Department of Chemical Engineering, MS Ramaiah Institute of Technology, Bangalore-560054, India | |
| 3Department of Chemical Engineering, JNTU College of Engineering, Anantapur-515002, India | |
| 4Department of Biotechnology, College of Science & Technology, Andhra University, Visakhapatnam-530003, India | |
| Corresponding Author : | Siva Kiran RR Department of Biotechnology MS Ramaiah Institute of Technology Bangalore-560054, India Tel: 0091-80- 23603123 Fax: 0091-80-23603124 E-mail: reddykiran.rsr@gmail.com |
| Received January 18, 2012; Accepted March 13, 2012; Published March 15, 2012 | |
| Citation: Siva Kiran RR, Madhu GM, Satyanarayana SV, Bindiya P (2012) Bioaccumulation of Cadmium in Blue Green Algae Spirulina (Arthrospira) Indica. J Bioremed Biodegrad 3:141. doi: 10.4172/2155-6199.1000141 | |
| Copyright: © 2012 Siva Kiran RR, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
In the present paper, toxic effects and bioaccumulation of cadmium (II) ions by blue-green algae Spirulina (Arthrospira) indica were discussed. Lab scale open race way ponds (0.5 m long, 0.4 m wide and 0.075 m deep) with Zarrouk medium and S. (Arthrospira) indica culture was loaded with various initial cadmium (II) ion concentrations (1-10 mg/L). The growth of S. (Arthrospira) indica was monitored for eight days. The growth of S. (Arthrospira) indica was adversely affected by Cd (II) at high concentrations (>1 mg/L), however at low concentrations upto 1 mg/L, Cd (II) could stimulate its growth slightly. The toxic effect of cadmium (II) ions was modeled by assuming Monod growth kinetics with competitive inhibition. The percentage decrease in the S. (Arthrospira) indica biomass was found to be 54.32% at 4 mg/L Cd (II) after 7 days. The lethal concentration and time (LCt50) of Cd (II) ions for seven days was measured as 3.61 mg/L. The organism was found tolerant up to 1 mg/L of Cd (II) and can have potential applications in removal of low concentration of Cd (II) ions from contaminated waters. The biosorption experiment was conducted at various initial Cd (II) concentrations (1-4 mg/L) with live S. (Arthrospira) indica . The biosorption equilibrium time was found to be 6 min. It had been observed that at the initial stage (0-5 min) the adsorption rate was so rapid that 80% of the metal had been biologically adsorbed. The experimental equilibrium data was verified using Langmuir and Freundlich adsorption isotherms. The maximum biosorption capacity of live S. (Arthrospira) indica was estimated to be 66.97 mg Cd (II) per gdw of S. (Arthrospira) indica . The study provides a new insight into the impact of cadmium (II) ions on S. (Arthrospira) indica , especially with regard to its toxicity, bioaccumulation and bioremediation potential.
| Keywords |
| Monod kinetics; Bioaccumulation; Cd (II); Raceway ponds |
| Introduction |
| The contamination of the environment with toxic chemicals is one of the major problems faced by all nations. Cadmium is the toxic element commonly found in industrial workplaces. It is extensively used in electroplating, painting and battery industries. Due to its low permissible exposure limit (PEL), over exposures may occur even in situations where trace quantities of cadmium are found. In spite of the increasing amount of research published on the interaction of microorganisms with heavy metals like cadmium, few works describe this interaction with Spirulina (Arthrospira) indica. The mechanism of heavy metal ions binding and toxic effect caused by them differ between microbial species and heavy metal ions. Heavy metal ions are usually transported via protein carriers into the cellular interior. The quantity of metal ion transferred into the cellular interior also depends on the concentration of metal ion in the proximity of transporting channel. The mechanism of bioaccumulation of heavy metal ions by microorganisms is still not completely understood. Chojnacka and Wojciechowski [1] described the two stage mechanism (active and passive stage) based on the two step bioaccumulation process; 1) Preliminary, quick binding of metal ions to cellular wall; 2) slower transport through cellular membrane. The growth of algae cultivation is more dependent on light, when compared with the nutritional contents. Many models are derived from a combination of light attenuation phenomena and a light response curve based on Beer-Lambert’s law and a Monod-type equation, respectively. Kim et al. [2] and Bagus [3] applied light dependent Monod equation for cyanobacteria in photo- bioreactor. Bioaccumulation experiments are conducted at optimal light intensities and the models based on deviation of light intensity cannot be applied. |
| Biological materials have been proved to be capable of adsorbing heavy metals from aqueous solutions, especially for the metal concentration below 1 mg/L. According to World Health Organization [4], USA, cadmium concentrations in unpolluted natural waters should be below 0.001 mg/L [5]. Murugesan et al. [6] reported the biosorption of cadmium by live and immobilized cells of Spirulina platensis. Bioremediation potential of Spirulina platensis was also studied by various researchers with almost all heavy metal ions and other toxic compounds [7-9]. Very few studies are reported using Spirulina maxima and Spirulina indica. Most of these biosorption and bioaccumulation studies on Spirulina platensis or Spirulina maxima are done in shake flask at restricted conditions. Commercial production of Spirulina biomass is done using large raceway ponds. |
| The present paper deals with bioaccumulation and biosorption studies of Cd (II) ions using Spirulina (Arthrospira) indica in small pilot scale open raceway ponds. The second step of bioaccumulation process described by Chojnacka and Wojciechowski [1] was modeled using Monod growth kinetics with toxic inhibition. Monod model was developed by assuming the growth limiting substrate as NaHCO3 and growth inhibitor/toxin as Cd (II). The selection of NaHCO3 as growth limiting substrate is purely based on its high concentration in the growth media (Zarrouk’s medium), when compared with other medium constituents. The percentage decrease in the Spirulina (Arthrospira) indica biomass and lethal concentration and time (LCt50) of the toxin (Cd (II)) dosage required to kill half the members of a tested population after seven days of test duration, was calculated. The biosorption potential of live S. (Arthrospira) indica to biologically remove cadmium (II) ions at low concentration (below 2 mg/L) was examfined. |
| Growth Kinetics |
| Growth of cells is described with Monod equation in the case of limiting concentration of substrates. Monod equation was used previously to describe growth rate of cells: |
 (1) (1) |
| Where X is the concentration of organisms (gdw/L) and μ is the specific growth rate (day-1). The idea of growth kinetics has been dominated by an empirical model originally proposed by Monod [10,11]. The Monod model introduced the concept of a growth limiting substrate. |
 (2) (2) |
| Where μmax = maximum specific growth rate, S = Substrate concentration (mg/L), Ks = substrate saturation constant (i.e. substrate concentration at half μmax) |
| Models for inhibition of growth by toxic compounds |
| The presence of toxic compounds in the growth medium can inhibit the growth of cells or even lead to cell death. The Monod kinetics can be applied by considering two types of growth inhibition equations |
Competitive inhibition  (3) (3) |
Non-competitive inhibition  (4) (4) |
| Ks, KI and μm are Monod constants, I = inhibitor concentration (mg/L), S = Substrate concentration (mg/L) and μg = Specific growth rate (day-1). |
| Shuler and Kargi [12] proposed a simple method for finding the actual inhibition in the microbial systems. If Ks increases as the toxic concentration increases then the system follows competitive inhibition (Equation 3). If the Monod kinetics parameter Ks is constant for all toxic concentrations, then the system confirm to follow Non- competitive inhibition mechanism (Equation 4). The present study was found to follow competitive inhibition and the kinetic parameters were calculated and tabulated at various Cd (II) ion concentrations. |
| Biosorption isotherms |
| The Langmuir sorption model was chosen for the estimation of maximum cadmium (II) sorption by live Spirulina (Arthrospira) indica. The Langmuir isotherm can be expressed as |
 (5) (5) |
| Where, Qmax is monolayer adsorption capacity of adsorben t (mg/g), b (L/mg) is the Langmuir constant and Cf (mg/L) is equilibrium concentration of Cd (II) [13]. |
| The Freundlich model is represented by the equation |
| |
| Where, K (mg/g) is the Freundlich constant related to adsorption capacity of adsorbent [13]. |
| Materials and Methods |
| Microorganism and media composition |
| Spirulina (Arthospira) indica was procured from Center for Advanced Studies, University of Madras, Chennai, Tamil nadu, India. Zarrouk’s medium [14] was used for the growth of Spirulina (Arthrospira) indica. The alga seed was first centrifuged and then stored in liquid medium (table 1) for 7 days at 20~26&°C under light generated by a 40 W white fluorescent lamp. The quantity of living biomass was found to be 0.12 gram dry weight of S. (Arthospira) indica cells in 3 L [8]. |
| Culture conditions |
| Experiments were carried out in a greenhouse in PVC open raceways (0.5 m long, 0.4 m wide, 0.075 m deep) containing 3 L of S. (Arthrospira) indica culture with an initial biomass concentration of 0.1 g/L. The cultures were mixed using paddle wheels turning at 18 revs/min and illuminated with daylight-type 40 W fluorescent lights (Philips, India) at an intensity of 1,900 lux and a 12 h light/dark photoperiod at 30Ã?Â?C [15]. |
| Quantification of S. (Arthrospira) indica biomass and NaHCO3 |
| Cell concentration was determined with a UV-visible recording spectrophotometer (Shimadzu, Japan) at a wavelength of 560 nm (the value independent on pigment concentration). The biomass were filtered immediately through prewashed and pre-weighed paper filters (Whitman No 2), and cell dry weights were also determined after drying filters at 80Ã?Â?C until the samples reached the constant weight. The growth limiting substrate (NaHCO3) concentration was determined by titrating with 0.2 M HCl using methyl orange indicator. |
| Acute toxicity tests |
| Growth inhibition was achieved by adding log phase S. (Arthrospira) indica culture (1:10) to six lab scale raceway ponds containing aqueous cadmium (II) ions of 0, 1, 2, 3, 4 and 10 mg/L with Zarrouk’s Medium [14]. The working volume of each pond was 3 L. The growth rate was determined every 24 h for eight days [16]. The depletion of the growth limiting substrate (NaHCO3) concentration was also determined every 24 h. Lethal concentration and time (LCt50) of Cd (II) ions and percentage decrease in the S. (Arthrospira) indica biomass was also calculated. |
| Biosorption experiments |
| The Cd (II) solution was prepared by diluting standard CdCl2 solution to the desired concentrations. The freshly diluted solutions were used for each biosorption study. The sorption experiments were conducted in lab scale raceway ponds (see culture conditions section) containing 3 L of cadmium solutions, with initial concentration 1 mg/L for 24 hours. After finding the equilibrium time from the first experiment, further experiments were carried out at initial concentrations ranging from 1 mg/L to 10 mg/L for 6 min. The pond was maintained at ambient temperature (30Ã?Â?C) and optimal light intensity. At the designed period of time, 5 ml of the solution was collected for analysis. To determine the concentration of the metal ions, the culture in the sample solutions was removed by filtration and the filtrate was analyzed to measure the cadmium (II) concentration using atomic absorption spectrophotometer (GBC Avanta Ver 1.32, Australia). |
| Results and Discussion |
| Toxicity of cadmium (II) ion on Spirulina (Arthospira) indica |
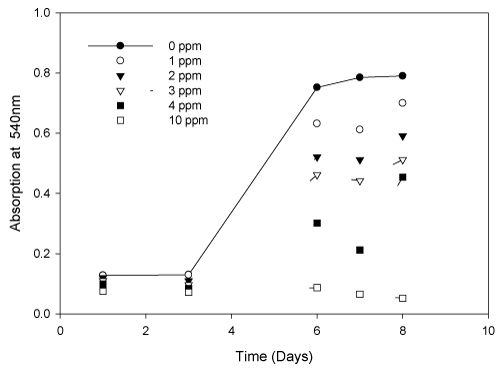
| To investigate Spirulina (Arthospira) indica tolerance of Cd (II) ions, the living cells were cultivated in solution containing Cd (II) with various concentrations (0, 1, 2, 3, 4, 5 and 10 mg/L). The growth curves of S. (Arthrospira) indica were shown in Figure 1. |
| It was evident from the above diagram that cadmium (II) concentration of 10 mg/L is highly toxic for the growth of S. (Arthrospira) indica. The results also indicated that the growth inhibition increased at higher aqueous cadmium (II) concentrations. Slight inhibition of cell growth occurred at low Cd (II) concentrations. S. (Arthrospira) indica could be used to remove Cd (II) ions from low concentration contaminated waters. The percentage decrease in the S. (Arthrospira) indica was tabulated (Table 2). The lethal concentration and time (LCt50) was also calculated to be 3.21 mg/L at 7 days. |
| Bioaccumulation kinetics |
| Microbial growth in the medium containing no Cd (II) ions was described with the following monod equation (2): |
 |
| Where S = Substrate concentration (g/L) and μ = Specific growth rate (day-1) |
| At various Cd (II) ion concentrations, I = 0.5,1, 2, 3 and 4 mg/L, the Monod growth kinetics parameters were estimated. The KS values were found to increase with increase in the Cd (II) ion concentrations up to 1 mg/L and μm values were found to be constants confirming the model to be competitive inhibition. Further, increase in the cadmium (II) ion concentration beyond 1 mg/L, the KS values were found to be increasing but μm values were unpredictable leading to unknown kinetics. At lower concentrations (<1 mg/L), Cd (II) may help in the growth of the Spirulina (Arthospira) indica similar to the sodium ions present in the growth limiting substrate (NaHCO3). Further increase in the cadmium (II) concentration resulted in toxicity and inhibited the growth. The competitive inhibition may take place between cadmium and sodium ions for the growth of the cells at concentrations below 1 mg/L Cd (II). Concentration above 1 mg/L cadmium (II) ions was more dominant than sodium leading to extreme toxicity, reducing the growth rate [12] (Table 3). |
| Biosorption of cadmium (II) ions in raceway ponds |
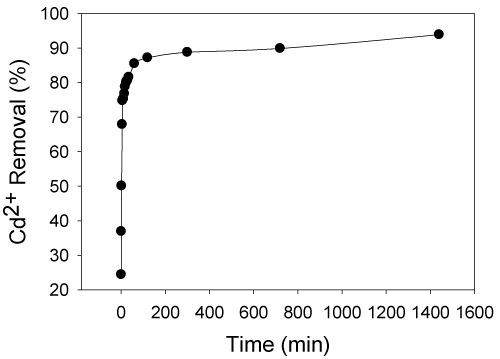
| The effect of time on cadmium (II) biosorption using live S. (Arthrospira) indica is shown in Figure 2. S. (Arthrospira) indica was grown in the Zarucks medium (table 1) containing 1 mg/L of Cd (II) ions. The percentage removal of cadmium (II) ion concentration was found at various intervals in the biosorption process. Rapid biosorption was observed at the beginning (1 mg/L) with 6 min and with 74% of cadmium (II) adsorbed, then reached the equilibrium within 1400 min (24 h) with 93.9% of metal ions adsorbed. Such rapid uptake of heavy metals by living cells was very significant when the cells were used in bioremediation process [8]. Experimental results matched with the standards recommended by World Health Organization (0.015 mg/L, cadmium concentration after 24 hours). The equilibrium time was found to be 6 minutes and further process parameters were evaluated at 6 minutes. |
| Biosorption equilibrium studies |
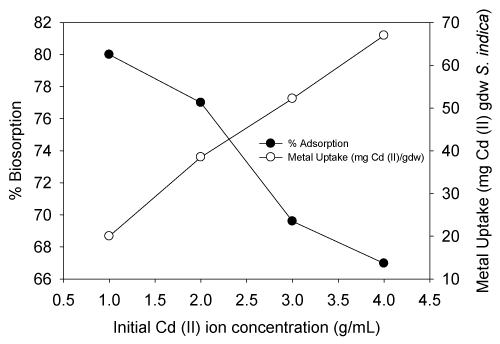
| The equilibrium biosorption of cadmium (II) ions on the live S. (Arthrospira) indica as a function of the initial concentration of cadmium (II) is shown in Figure 3. There was a gradual increase of adsorption for cadmium (II) ions until equilibrium was attained. The Langmuir and Freundlich models were often used to describe equilibrium sorption isotherms. The calculated results of the Langmuir and Freundlich equations with constants were shown below. The percentage adsorption gradually reduced as the Cd (II) ion concentration increased and metal uptake increases with increase in Cd (II) ion concentration. |
| The linearized Langmuir equation (6) and Freundlich equation (7) were found to be |
 (6) (6) |
| |
| The adsorption of cadmium on the S. (Arthrospira) indica was correlated well with the Freundlich equation (R2 = 0.99736615) as compared with the Langmuir equation (R2= 0.96764868). |
| Such decline in percentage adsorption of cadmium (II) ion is probably caused by the saturation of some adsorption sites on live S. (Arthrospira) indica (Figure 3). When the Cd (II) concentration was increased from 1 to 4 mg/L approximately, the metal uptake increased from 20 to 66.79 mg Cd (II) per gdw S. (Arthrospira) indica cells after 6 min equilibrium time of adsorption. The maximum adsorption capacity was found to be 80% at 1 mg/L initial concentration. |
| Conclusion |
| In the present work two different models, Monod kinetics with growth limiting substrate with toxic compounds and biosorption isotherms were presented. The first model is purely based on the analysis of the process mechanisms of the actual bioaccumulation. Kinetic model however makes it possible to analyze various aspects of bioaccumulation. The second model is based on the quick, preliminary accumulation of Cd (II) ions on the surface of S. (Arthrospira) indica cells. This study led to the conclusion that bioaccumulation of heavy metal cadmium (II) follows monod kinetics with competitive inhibition mechanism at low concentrations (<1 mg/L). The S. (Arthrospira) indica had low (3.21 mg/L Cd (II) at 7 days) lethal concentration and time (LCt50) indicating the toxicity of Cd (II) ions at high concentrations (>3.21 mg/L). In addition, living cells of S. (Arthrospira) indica were found high tolerance to cadmium (II) at low concentration and can be regarded as an attractive adsorbate option for the biosorption of cadmium contaminant. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 15933
- [From(publication date):
March-2012 - Dec 04, 2025] - Breakdown by view type
- HTML page views : 10995
- PDF downloads : 4938
