Research Article Open Access
Assessing Bioremediation of Acid Mine Drainage in Coal Mining Sites Using a Predictive Neural Network-Based Decision Support System NNDSS)
| Xiaoci Ji1, Steven A Ripp2, Alice C Layton2, Gary S Sayler2,3, Jennifer M DeBruyn1* | |
| 1Department of Biosystems Engineering and Soil Science, The University of Tennessee, USA | |
| 2Center for Environmental Biotechnology, The University of Tennessee, USA | |
| 3Department of Microbiology, The University of Tennessee, USA | |
| Corresponding Author : | Jennifer M DeBruyn Department of Biosystems Engineering and Soil Science The University of Tennessee, USA Tel: 865-974-7266 E-mail: jdebruyn@utk.edu |
| Received September 05, 2013; Accepted October 04, 2013; Published October 10, 2013 | |
| Citation: Ji X, Ripp SA, Layton AC, Sayler GS, DeBruyn JM (2013) Assessing Long Term Effects of Bioremediation: Soil Bacterial Communities 14 Years after Polycyclic Aromatic Hydrocarbon Contamination and Introduction of a Genetically Engineered Microorganism. J Bioremed Biodeg 4:209. doi: 10.4172/2155-6199.1000148 | |
| Copyright: © 2013 Ji X, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
In this study, an Artificial Neural Network (ANN) was developed as a predictive tool for identifying optimal remediation conditions for groundwater contaminants that include selected metals found at coal mining sites. The ANN was developed from a previous field data obtained from a bioremediation project at an abandoned mine at Cane Creek in Alabama, and from a coal pile run off at a Department of Energy’s site in Aiken, South Carolina. The evaluative parameters included pH, redox, nutrients, bacterial strain (MRS-1), and type of microbial growth process (aerobic, anaerobic or sequential aerobic-anaerobic conditions). Using the conditions predicted by the Neural Networks, significant levels of As, Pb, and Se were precipitated and removed over eight days in remediation assays containing 10 mg/L of each metal in cultures that include MRS-1. The results showed 85%, 100%, and 87% reductions of As, Pb, and Se, respectively. The results from these ANN- driven assays are significant. It provides a roadmap for reducing the technical risks and uncertainties in clean-up programs. Continuous success in these efforts will require a strong and responsive research that provides a decision support system for long-term restoration efforts.
| Keywords |
| Groundwater contaminant; Acid mine drainage (AMD); Artificial neural networks (ANN); Metals |
| Introduction |
| The extent to which microbial systems can be used in treatment of metals Acid Mine Drainage (AMD) sites varies with the species and may be complicated by the nature of both the absorbent and the metal species in aqueous solution. Therefore, strategies that involve the use of microbial processes will depend to a large degree on their ability to accumulate a variety of metal ions before the cells become affected by metal toxicity. |
| The chemical and biological reactions of pyrite in AMDs generate acidic minerals, which can oxidize to form sulfuric acid, ferrous sulfate, and associated toxic metals. Two major reactions have been noted to be responsible for the oxidation process [1-5]. |
| This is a complex reaction that begins with the oxidation of pyrite, and continues with the oxidation of iron (II) ion to Fe (III) ion |
| Since reactions (a) and (b) are pH sensitive, the rate law could be represented as: |
| The re-oxidation of ferrous ions in reaction (b) can be accelerated by sulfur-oxidizing acidophilic bacteria such as Acidithiobacillus ferrooxidans and other acidophilic microbes [4]. |
| The Fe3+ from reaction (b) further dissolves pyrite |
| This in combination with reaction (b) forms a cyclic reaction for the dissolution of pyrite to form iron (III) precipitates as hydrated iron (III) [1] |
| Castro et al. [6] had noted that in the presence of air and water, it is possible to generate various insoluble ions such as Fe (S04)3. H2O (ferrous sulfate), FeSO4.7H2O (Melanterite) and (FeS04)3.9H2O (Coquimbite). |
| The primary intent of this proposal is to mitigate the reoxidation of ferrous ions by promoting the growth of ferrous-sulfate dependent bacterial strains. |
| Passive Treatment Systems |
| The total cost of cleaning up pollution from AMD sites is difficult to quantify. The US EPA identified 156 abandoned mine sites that were on or had potential to be on the National Priorities List (NPL) for remediation under the Comprehensive Environmental Response, Compensation and Liability Act (CERCLA), with the potential to cost between $7 and $24 billion to clean up [7,8]. Regions where soils are relatively acidic and low in carbonates offer the least attenuation [9] Chemical processes for the treatment of mine water are expensive and typically result in high quantities of inorganic sludge materials for disposal. Thus, there are significant opportunities for biological treatment efforts with enhanced strategies for metal recovery [10-13]. The cost challenge also presents opportunities for re-assessing the processes in passive treatment such that the roles and functions of the microbial communities are fully utilized for enhanced treatment. Currently, the basic design for passive treatment systems involves chemical or biological acid neutralization and metals removal. Some examples are described: (a) Aerobic Wetlands [14-16]. The shallow configuration of this system encourages emergent vegetations, with the goal of promoting the oxidation of Fe and Mn, and the co-precipitation of metals [8,11]; (b) Anoxic Limestone Drains (ALD). In this is system, mine water flows through limestone channel under anoxic conditions. The process promotes alkalinity and prevents limestone armoring. Fe is precipitated accordingly [12,13]; (c) Open Limestone Channels (OLC). Similar to ALD, alkalinity is promoted, with the precipitation of Al, Fe, Mn as metal oxides [13]; (d) Successive Alkalinity Producing Systems (SAPS). This falls under the vertical flow systems, which allow mine water to drain through layers of limestone and anaerobic organic matter. The system promotes alkalinity, sulfate reduction and metal precipitation; (e) Anaerobic Wetlands [17-19]. This subsurface system is designed to be isolated from atmosphere by standing water or overlying material. The system promotes alkalinity; sulfate reduction and precipitation of metal sulfides; and sorption or uptake by vegetation [20,21]; (f) Sulfate-Reducing Bioreactors. Mine water drains into anoxic chamber containing organic matter and sulfate- reducing bacteria. The system also promotes alkalinity, sulfate reduction, metal precipitation, and sorption [22-24] and (g) Amendments. This is an alternative approach to passing mine water through a treatment system, and designed to perform in situ treatment, by adding amendments to standing water, soil, tailings piles, or exposed rock surfaces. The system may serve multiple purposes, such as revegetation and soil stabilization, acid neutralization, contaminant immobilization, or stimulation of microbial-mediated alkalinity addition and metals removal [3,25,26]. |
| Role of Biotechnology in AMD Mitigation |
| Microorganisms can be involved in AMD abatement, primarily through the reduction of metals and sulfates, as well as other alkalinity generating processes [11,12]. The extent to which each process may contribute to the neutralization of AMD depends upon the chemical composition of AMD, the availability of necessary electron donors/ receptors, temperature, and pH within the mine-waste environment. Acidophilic heterotrophic bacteria present in the AMD environment may be involved in AMD that are potentially toxic to iron-oxidizing bacteria, thereby, inhibiting biologically mediated iron oxidation reactions [1]. Other species demonstrated the ability to reduce the Fe present either as soluble or as solid-phase compounds to ferrous iron. |
| Mn and Fe may also contribute to the neutralization process. Microorganisms, including the heterotrophic bacteria Pseudomonas, Clostridium, and Desulfovibro, can directly reduce Mn and Fe by using them as final electron acceptors under anaerobic conditions. The ability to oxidize ferrous ion is widespread among acidophilic heterotrophic bacteria and has been reported for Acidithiobacillus ferrooxidans growing on elemental sulfur. When ferric ion is reduced to ferrous iron, the removal of iron from AMD becomes easier, because ferrous iron reacts with sulfide produced by sulfate reduction, and this ultimately, results in the removal of Fe and promotes alkalinity. Sulfate reduction leads to permanent alkalinity generation. Sulfate reduction leads to permanent alkalinity production when H2S gas is released from mine waste environment. |
| Other biologically mediated process that can contribute to AMD neutralization by ultimately consuming H ions include: ammonification by various microorganisms; denitrification, where a number of bacteria species, such as Pseudomonas, Paracoccus, Flavbacterium, Alcaligens, and Bacillus spp., convert ammonia to nitrates under anaerobic conditions; and methane generation by methanogenesis. |
| Microbiological Sulfate Reduction |
| A group of bacteria called Sulfate Reducers (SRB), such as Desulphovobrio spp. can convert sulfate contained ARD to sulfide and can generate bicarbonate in the presence of organic carbon nutrient sources using it as an electron donor under anoxic and reducing conditions. Sulfate reduction first produces HS. The HS generated forms insoluble metal complexes and results in the removal of metals such as Fe. The bicarbonate released results in an alkalinity |
| |
| Fe + FeS |
| SRB are known to be natural soil bacteria and can be found in soils. They require low-molecular organic carbon compounds (e.g. simple organic acids), suitable concentrations of sulfate (0.200 mg/l), pH level greater than 4.5, and low Eh (-150 mV). SRB can function in the absence of oxidizing agents such as 02 and Fe3+. Low- molecular weight carbon compounds (e.g. lactic acid and acetate) used by SRB are common products of natural degradation (i.e. microbial fermentation) processes, which occur in anoxic environments [27,28]. A variety of materials, depending on their cost and availability, can be used. They may include industrial wastes such as molasses, sewage sludge, compost, and manure. The materials can be supplemented with materials containing nitrogen and or phosphorus to obtain the optimal nutrient composition required. The pH requirements are obtained by the alkalinity generated by microbial activity and carbonate dissolution. |
| The overall process results in an improvement in water quality due to the precipitation of metals as sulfides, with the H2S generated in organic substrates and neutralization of the acidity due to the bicarbonate released during sulfate reduction. |
| Experimental |
| Designing and training methods of the artificial neural networks |
| An Artificial Neural Network (ANN) is a mathematical simulation of the neurological functioning of the brain. The ANN consists of neurons and connections between the neurons corresponding to inputs similar to the way the brain functions. The neurons are called “nodes” that are grouped in layers. A multilayer neural network consists of (1) an input layer, (2) a number of hidden layers, and (3) an output layer. The nodes in each layer are connected to the nodes of the next layer in ANN. Training of the network involved presenting the ANN with inputs data with known output. The ANN learns the pattern by adjusting the weights of the connections. During the training of ANN, the weights are adjusted until the error between the actual output and the predicted output is minimum. An optimal ANN design in terms of number of nodes and layers is usually designed with different combinations of nodes and layers several times. |
| The ANN was built using field data from a previous project conducted by Prof. Ibeanusi and his research team at Cane Creek Coal Valley Site, in Alabama, and at a Department of Energy’s Savannah River Coal Pile run off Site [9]. Approximately 80% of the data sets were used as training subsets. About 20% of the data was used as validation and monitoring subsets. After evaluation of complete training set, the overall network performance was assessed using the monitoring set. Commands in MATLAB were used to create models of the ANN to run efficiently. The methodology developed to design ANN with high accuracy is based on the neural network conceptualization. A set of commands were used to depict the training inputs and targets, to create and train the ANN, and to predict a new set of inputs (table 1). |
| A Graphical User Interface (GUI) was developed using MATLAB. The GUI facilitated in studying the effects of temperature, aeration, treatment duration and ratios of nutrient, AMD and bacteria on the final pH of the bioremediation experiments. Profiles were predicted by varying one variable while keeping all other variables constant (Figure 1). |
| Water sample and metal analysis |
| Bacterial growth dynamics was determined with Spectronic Gensys 2 at 600 nm. Water samples and associated microbial biomass were initially digested with a CEM Microwave Digesting System (MDS 2000, CEM Corporation) and subsequently analyzed by Inductively Coupled Plasma (ICP) Spectrometry (Perkin Elmer 400, Covina, CA). All samples were acidified before ICP analysis. Metal concentrations were calculated as follows: |
| Metal concentration (mg l-1) |
| Metal treatment and removal experiments |
| In each triplicate culture containing 100 ml of mine water from a coal pile run off were inoculated with a mixture of mid log 106 cell ml-1 of each bacterial strain and compared with two sets of control flasks with no bacterial addition. One set of the control flasks contained wastewater only, while the second set had wastewater plus nutrients. This experimental set up allowed us to evaluate the role of the bacterial strains and the effects of the nutrients on the metal removal. |
| Growth nutrients (in grams per liter) were ammonium sulfate, ammonium nitrate, 0.5; potassium phosphate, 0.5; pyruvic acid, 1.2; oxalic acid, 1.2. Pyruvic and oxalic acids were added to specifically inhibit the growth of indigenous bacteria in the mine water, such as Acidithiobacillus ferrooxidans, and other acidophiles, which are usually associated with acid mine drainage. Culture flasks were grown at 35°C in a shaker agitated at 160 rpm for 21 days (Table 2). |
| Results and Discussion |
| The artificial neural networks |
| The objective of this project was to develop an ANN as a decision support system that serves as a tool to predict optimum bioremediation conditions for wastewater and surface water. The strategy is based on inputting field data into ANN to produce an optimum bioremediation model to effectively clean-up contaminated sites. The developed ANN was evaluated using field data from a remediation project of an acid mine drainage site, and from a coal pile run off. |
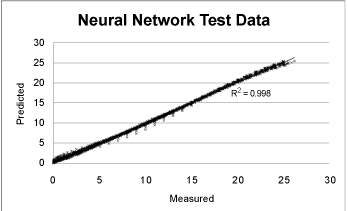
| The ANN was trained to adapt and to learn from a training subset of the field data. After training, the ANN was used to assess the ANN. Figure 1 shows a good correlation between ANN predicted values and measurements from the validation subset. These results show that the ANN approach to model bioremediation experiments is feasible. |
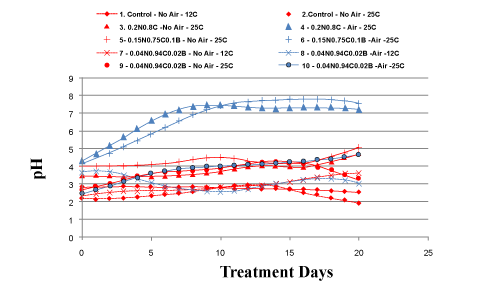
| After the ANN was developed, ANNOT was used to predict the pH of 14 profile datasets. Figure 2 shows the remediation profile over time. Each curve represents an initial remediation condition. Figure 2 shows that aeration (indicated by blue lines) and higher temperatures (indicated by continuous lines) promoted remediation. It shows that the red lines are stable and two blue lines increases to a higher stable pH level. On the other hand remediation is inhibited when no aeration is provided (indicated by red lines) and site temperature is low (indicated by dashed lines). In this case, dashed red lines tend to be on the bottom of the graph, while blue continuous lines tend to be on top. |
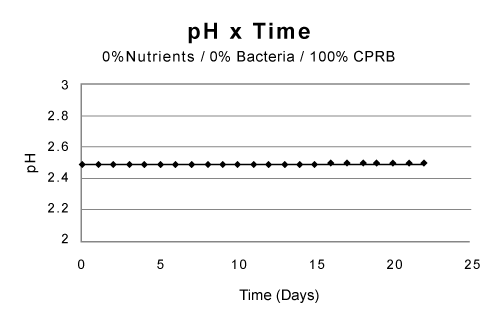
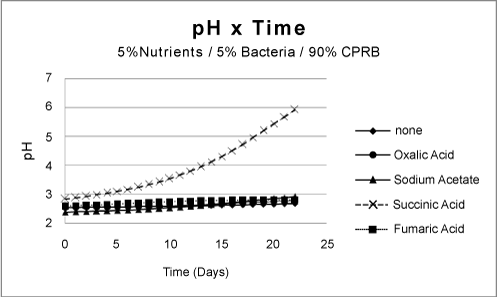
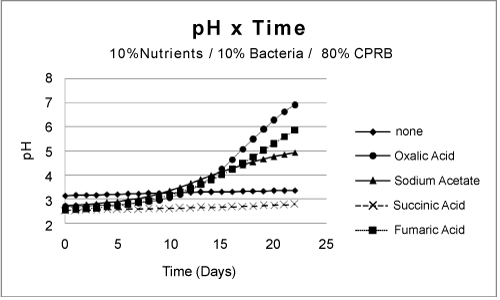
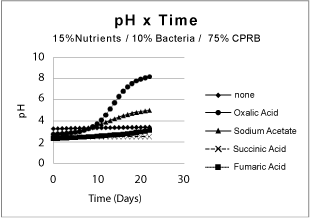
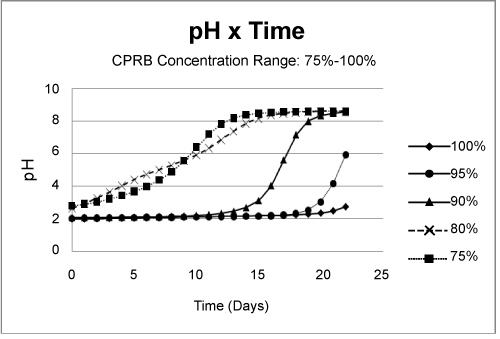
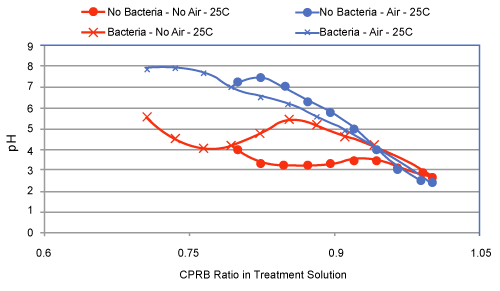
| Partial remediation of the metals was also possible in the absence of bacteria if the growth conditions were augmented with nutrients and aeration. Nevertheless, for best initial conditions (air, nutrient, bacteria, and temperature at 25ºC) provided an excellent condition for metal remediation (Figure 3-5). Remediation conditions were further investigated at 15 days for different treatment cultures. The results, presented in Figure 6, show that bioremediation can be achieved with CPRB or AMD ratio as great as 90%, as long as air is provided. If the amount of CPRB or AMD is greater than 90% it is difficult to achieve pH greater than 5. |
| Remediation of metals |
| Once ANN was optimized, the remediation conditions it described were tested in the laboratory by growing MRS-1 bacterium in the presence or absence of As, Se, and Pb, during an eight-day period. The growth of MRS-1 was studied by measuring the absorbance at 600 nm. The growth of the bacterium in the presence of As, Pb, and Se appeared to have a similar growth profile as bacterial strain without any metal indicating the tolerance of this bacterium in metals. The concentrations of As, Pb, and Se decreased daily during the experiment. |
| Summary |
| This project integrates microbiological processes into Artificial Neural Networks (ANN) technology as a strategy that provides a decision support system for reducing the technical risks and uncertainties in achieving enhanced treatment of acid mine drainage sites. Conventional passive treatment of mine water has primarily focused on constructed wetlands technology and their associated types of vegetations. One of the draw backs in these systems is that they tend to work well in mildly acidic water (pH < 4.5), and in more acidic water (< 3.5), they often would accumulate metals, especially dissolved aluminum, which over time, reduces the permeability of the anoxic limestone drains to the point of failure. The roles of microbes in these systems have not been fully explored, and their functions remain poorly understood. The bacterial culprit in these mine water reactions is Acidithiobacillus ferrooxidans, a chemolithothrophic bacterium that derives energy from oxidizing ferrous and sulfide ions to ferric and sulfate ions, respectively, with the resultant pH of 2-3. |
| Through this study, we have identified bacterial strains, which through nutrient manipulations are able to compete and out-grow A. ferroxidans in mine water, without the active re-oxidation of ferrous and sulfide ions to insoluble ferric ions and sulfate. Using this knowledge base, we have developed an Artificial Neural Networks from a previous field project that was collected from a project at abandoned mine at Cane Creek Surface Mining (OSM), we have accumulated promising data that supports the use of Artificial Neural Networks (ANN) as a useful predictive tool for assessing the remediation of acid mine drainage sites. |
| Our intent in this project is to incorporate the various nutrient augmentations and bacterial strains as additional inputs in the Neural Networks so that the outputs from the ANN could be optimized for enhanced mine water treatment. Specifically, the objectives of this proposal are to: (1) isolate, identify, and optimize the growth conditions of specific bacterial strains in mine water; (2) incorporate the growth conditions from Objective 1 to optimize our ANN; and (3) Use the output results from the ANN to demonstrate the treatment of acid mine drainage at a specific OSM site. As described, this proposal is designed to address one of the objectives of the National Technology Transfer Team (NTTT) of the OSM by promoting a broader understanding of and support for technology transfer in OSM (Figure 7,8). |
| In addition, the project supports one of the goals of the NTTT Applied Science Program by providing opportunities for minority institutions to participate in research projects related to coal mining in order to build the pool of a diverse workforce for the OSM. |
| Conclusion |
| The use of field data from a previous remediation project at Cane Creek, Coal Valley Site in Alabama, and from a coal pile run off at Department of Energy’s Savannah River Site to create an Artificial Neural Network was found to be an effective predictive tool for evaluating the efficiency and performance of remediation of acid mine drainage and groundwater contaminants. The project shows that the use of ANN is fast and could be used to predict optimum bioremediation conditions. Also, application of ANN is expected to reduce the time it takes to assess the bioremediation strategy to detoxify contaminants in both groundwater and surface water. The investigation of ANN identified optimal remediation conditions for metals. Future research will further improve ANN predictions for VOCs. |
| Acknowledgements |
| This work was funded by the Office of Surface Mining (OSM Award Number: S08AP12918). |
| Initial funding was received from the Department of Energy based on work conducted at Savannah River Site, Aiken, SC. Special thanks to Gustavo Menezes for his dedication and special contributions on artificial neural networks. My gratitude to Cassia de Brito, who served as the Co-Pi in the OSM grant. Field data for building the ANN were generated from work conducted by Prof Victor M. Ibeanusi and his team. |
| Disclaimer |
| This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
 |
 |
 |
 |
| Figure 5 | Figure 6 | Figure 7 | Figure 8 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 16367
- [From(publication date):
May-2012 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 11594
- PDF downloads : 4773
