 |
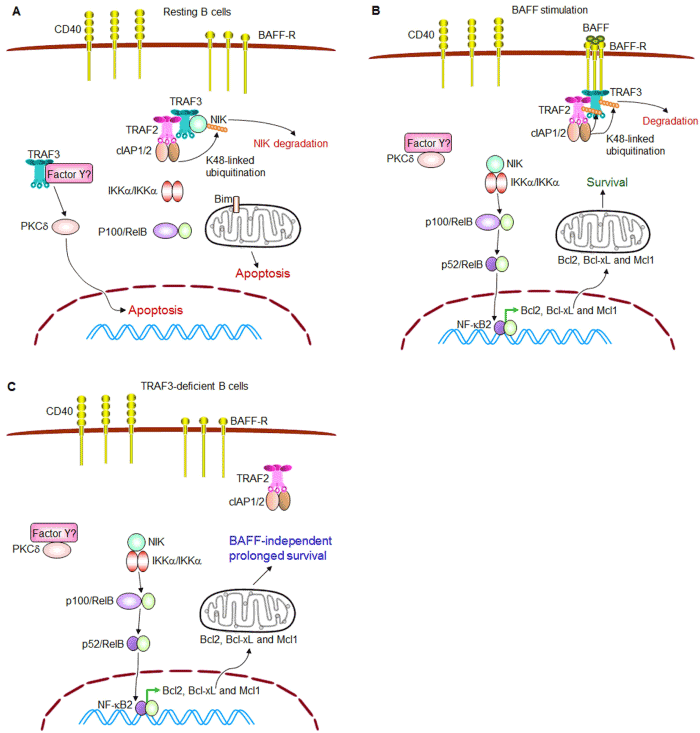
| Figure 1: TRAF3 and BAFF signaling pathways in regulating B cell survival. (A) TRAF3 promotes apoptosis in resting B cells. In the absence of stimulation, TRAF3 inhibits NF-κB2 activation while facilitating PKCδ nuclear translocation to promote B cell apoptosis. TRAF3 and TRAF2 constitutively form a complex with cIAP1/2 and NIK, targeting NIK for K48-linked polyubiquitination and degradation, thereby inhibiting NF-κB2 activation in B cells. How TRAF3 facilitates PKCδ nuclear translocation remains to be determined (depicted as through binding to an unknown protein or multi-protein complex Factor Y in the figure). (B) BAFF stimulates B cell survival. Upon ligand engagement, trimerized BAFF-R recruits TRAF3 and the associated TRAF2-cIAP1/2 complex to membrane rafts, and thus releases NIK, allowing NIK accumulation and NF-κB2 activation. Meanwhile, Factor Y is also released from TRAF3 and may sequester PKCδ in the cytosol. NF-κB2 activation together with reduced nuclear level of PKCδ functions to induce the expression of anti-apoptotic proteins, and thus promotes B cell survival. (C) TRAF3 deficiency causes BAFFindependent B cell survival. Similar to BAFF stimulation, deletion of TRAF3 from B cells (caused by either biallelic deletions or inactivating mutations of the Traf3 gene) also releases NIK from the TRAF2-cIAP1/2 complex, causing constitutive NF-κB2 activation. In the absence of TRAF3, Factor Y may also sequester PKCδ in the cytosol. Therefore, constitutive NF-κB2 activation together with reduced nuclear level of PKCδ leads to BAFF-independent, prolonged survival of TRAF3-/- B cells. |