 |
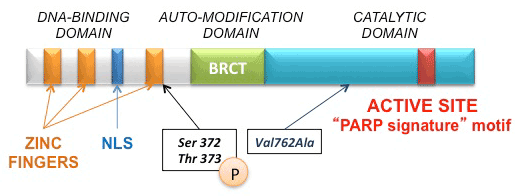
| Figure 2: PARP-1 proteins domains. PARP-1 has a carboxyl-terminal domain (catalytic domain) with an enzymatic activity in the “PARP signature” motif that catalyzes the cleavage of the coenzyme nicotinamide adenine dinucleotide (NAD+) into nicotinamide and ADP-ribose. PARP-1 also has an amino-terminal DNA binding domain containing three zinc finger motifs, a nuclear localization signal (NLS), and an auto-modification domain that functions as the target of covalent auto-poly(ADP-ribosyl)ation. The phosphorylation of PARP-1 at Ser 372 and Thr 373 residues is required for the maximal activation of the enzyme in response to DNA damage. PARP-inhibitors bind to the donor site of the “PARP signature” motif in the catalytic domain causing reversible inhibition of PARP enzyme. Val762Ala polymorphism in the catalytic domain represents the most frequent variant of PARP1, associated with an increased risk of many tumors. |