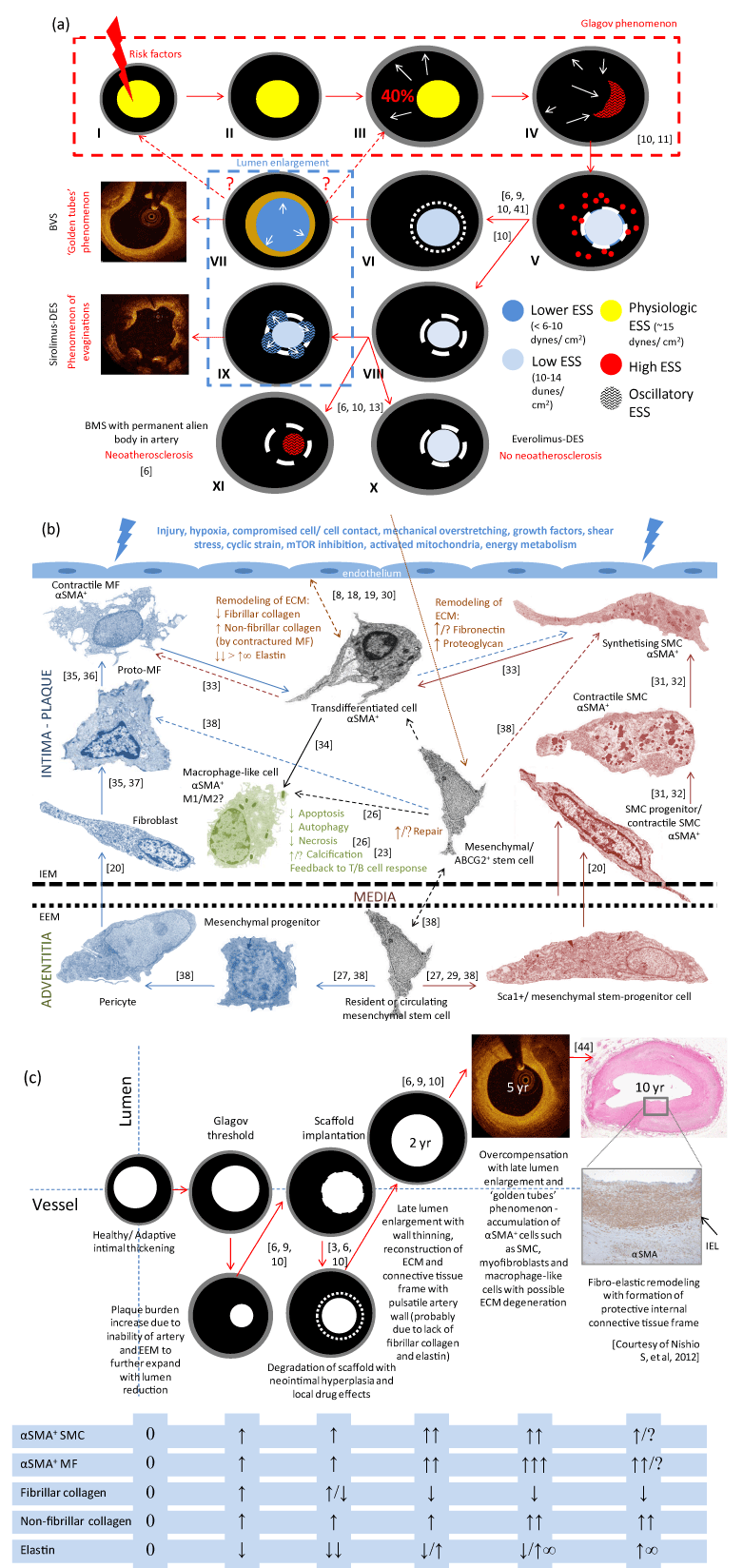
Panel (a) shows the general concept of the Glagov phenomenon (frames I-IV), and of the BRS-mediated reversal (frames V-VII). After BRS implantation, an artery undergoes the remodeling process with lumen enlargement, vessel wall thinning (plaque-media reduction) and pseudo-atheroregression (with OCT-visible ‘golden tube’), which can be regarded as a kind of vascular reparative therapy. At 24 months, most struts of BVS 1.0 ABSORB are no longer detectable. In contrast to BRS, a metal cage (usually sirolimus-DES or BMS; see frames V, VIII, XI) provokes chronic irritation of tissue with progressive neoatherosclerosis, or can prevent neoatherosclerosis (DES; see frames V, VIII, IX, X) with OCT-detectable coronary evaginations–defined as outward bulges in the luminal contour between struts (sirolimus-DES; see frame IX) fixed to the struts, limiting further artery wall expansion. Endothelial shear stress (ESS) adjusts to artery remodeling and transient scaffolding.
Panel (b) demonstrates the hypothetical cellular biology (animal data-based) of artery remodeling after the implantation of BVS/mTOR inhibitor platform. There are depicted: the role of phenotypic switch of smooth muscle cells (SMC) and fibroblasts/myofibroblasts (MF), migration (from the circulation, perivascular adipose tissue and resident sites–internal layers of adventitia) and action of different stem-progenitor cells (Sca1+, mesenchymal, circulating, bone marrow-derived) as well as activation of α-smooth muscle actin (+) (α-SMA) cells (MF, SMC, macrophages) with reconstruction of extracellular matrix (ECM) frame. IEM–internal elastic membrane, EEM–external elastic membrane. Pathways of α-SMA cell activation are considered as potential targets for the management of artery remodeling. Panel (c) schematically represents the reorganization of the ECM and related artery remodeling after BVS implantation with analysis of the vessel/lumen ratio over time. Accumulation of α-SMA(+) cells with degradation of fibrillar collagen and deposition of elastin with non-fibrillar collagen play a major role in late lumen enlargement. IEL–internal elastic lamina. FIGURE ADAPTED FROM REFERENCE 2, 5, 7, 20.