 |
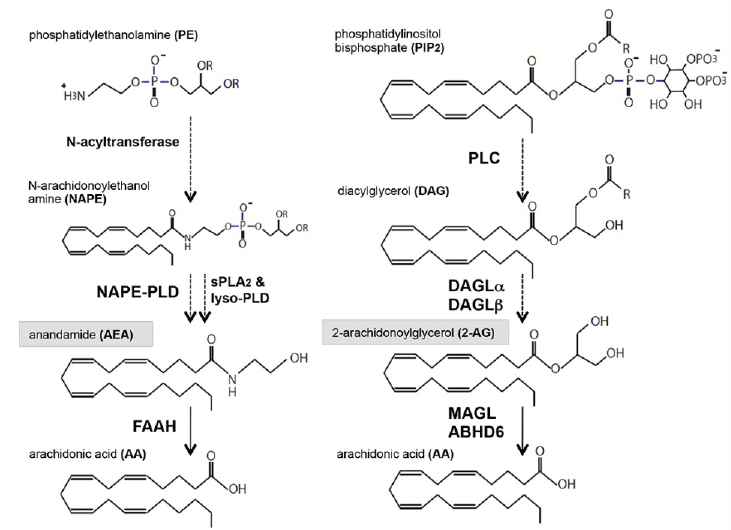
| Figure 1: Endocannabinoid (eCB) biosynthesis and hydrolysis. N-arachidonoylethanolamine (AEA) biosynthesis is initiated by the formation of N-arachidonoyl phosphatidylethanolamine (NAPE), which is formed by the transfer of arachidonic acid (AA) from phosphatidylcholine (PC) to phosphatidylethanolamine (PE) by N-acyltransferase (Step 1). Multiple synthetic pathways of AEA are postulated from NAPE, as indicated by NAPE-phospholipase D (NAPE-PLD) catalysis or successive multi-step reactions including secreted phospholipase A2 (sPLA2) and lyso-PLD (Step 2). AEA is mainly degraded by fatty acid amid hydrolase (FAAH) in the brain (Step 3). 2-arachidonoylglycerol (2-AG) is produced by the hydrolysis of sn-2 arachidonoyl phosphatidylinositol 4,5-bisphosphate by phospholipase C (PLC) and subsequent cleavage of the generated diacylglycerol (DAG) by sn-1-specific diacylglycerol lipases (DAGLα and DAGLβ) (Steps 1 and 2). Monoacylglycerol lipase (MAGL) degrades most of the 2-AG in the brain, and α/β hydrolase domain-containing protein 6 (ABHD6) also catalyzes the hydrolysis of the residual 2-AG at the postsynaptic compartment (Step 3). |