 |
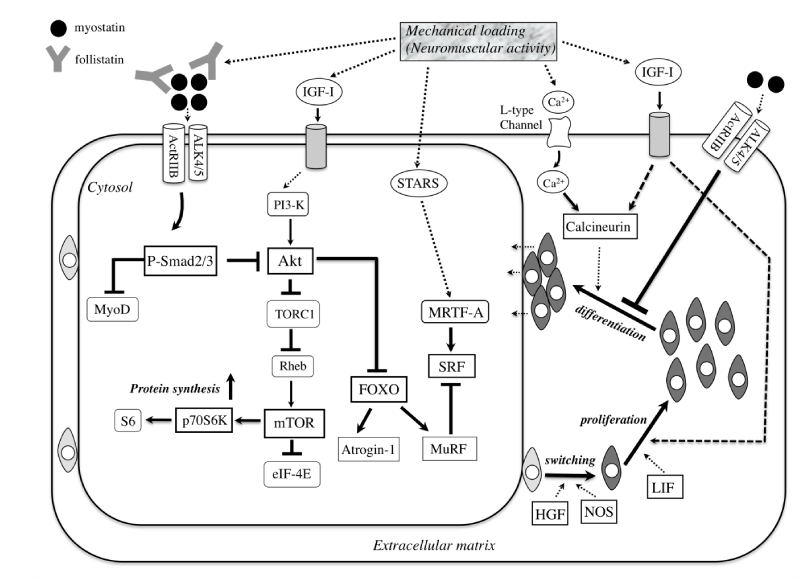
| Figure 1: Myostatin signals through the ActRIIB-ALK4/5 heterodimer activate Smad2/3 with blocking of MyoD transactivation in an autoregulatory feedback loop. In addition, Smad3 sequesters MyoD in the cytoplasm to prevent it from entering the nucleus and activating the stem cell population. Recent findings [79,80] suggest that myostatin-Smad pathway inhibit protein synthesis probably due to blocking the functional role of Akt. Mechanical loading (neuromuscular activity) upregulates the amount of follistatin, a myostatin blocker, and of IGF-I and then stimulates protein synthesis by activating Akt/mTOR/p70S6K pathway. Akt blocks the nuclear translocation of FOXO to inhibit the expression of Atrogin-1 and MuRF and the consequent protein degradation. Myosin-actin interaction by mechanical loading activate STARS / MRTF-A/SRF signaling [124]. In contrast, accumulation of MuRF in muscle tissue under inactivity (hindlimb suspension, immobilization, etc) inhibits SRF-dependent transcription of muscle-specicfic genes [122]. HGF and nNOS co-ordinately regulate switching of satellite cells from quiescence to activation. IGF-I and LIF enhance proliferaion of satellite cells. After stimulating by both Ca2+ and IGF-I, calcineurin promote the differentiation of these cells through MyoD, myogenin, and MEF2 [101]. These calcineurin’s function during differentiation is abrogated by myostatin-dependent signaling. In hypertrophic muscle after mechanical overloading, the differentiating myotubes seems to be incorporated to the existing muscle fibers ultimately. ActRIIB; activin receptor IIB, ALK4/5; activin-like kinase 4/5, eIF4E; eukaryotic initiation factor 4E, FOXO; Forkhead box O, HGF; hepatocyte growth factor, IGF-I; insulin-like growth factor-I, LIF; leukemia inhibitory factor, MRTF-A; mycardin-related transcription factor-A, mTOR; mammalian target of rapamycin, MuRF; muscle ring-finger protein, NOS; nitric oxide synthase, PI3-K; phosphatidylinositol 3-kinase, Rheb; Ras homolog enriched in brain, SRF; serum response factor, STARS; striated muscle activators of Rho signalng, TORC1; a component of TOR signaling complex 1 |