 |
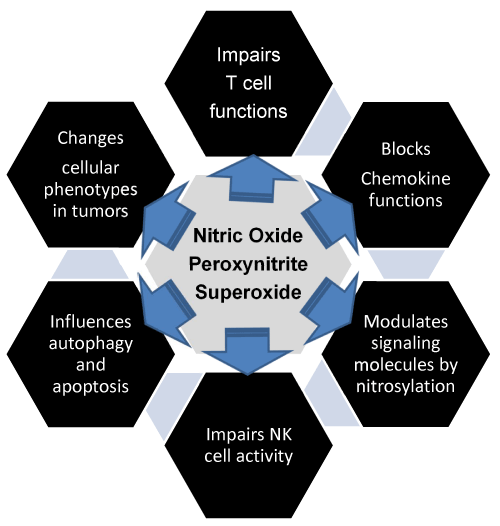
| Figure 1: Nitric oxide, peroxynitrite, and superoxide promote immunosuppression in tumor milieu: Most tumors show increased levels of free radicals which include reactive nitrogen and or oxygen species; however, cancer cells disregard the availability of oxygen and choose a less efficient path for generating energy. Cancer cells operate via the pentose phosphate pathway (PPP) for their energy which generates fewer free radicals as compared to the glycolysis. The aim is to prevent the buildup of reactive nitrogen species and reactive oxygen species and oxidative damage. In the process, cancer cells show increased levels of superoxide dismutase (SOD) activity and hypoxia-inducible protein factors (HIFs). HIF-1α is a transcription factor which induces transcription of several genes in hypoxia and low oxygen tension for cell survival. SODs are responsible for eliminating free radicals. The reactive nitrogen and or oxygen species support conditions of immunosuppression in the tumor microenvironment. Some of the mechanisms include modification of metabolites of transformed cells and other cells in the vicinity such as impairing the activities of T and NK cells that are involved in tumor regression. Reactive nitrogen species modify chemokine that are required for signals that facilitate the entry of effectors cells into the tumor but allow myeloid cells which promote immunosuppression. Nitric oxide modifies various signal transduction molecules within the cells that alter the functions of various cells with respect to autophagy and apoptosis. It also modulates oncogenes like p53 which regulate apoptosis [7]. Reactive nitrogen and or oxygen species influence the changes in the cell-surface markers and modify cell phenotypes involved in carcinogenesis. |