 |
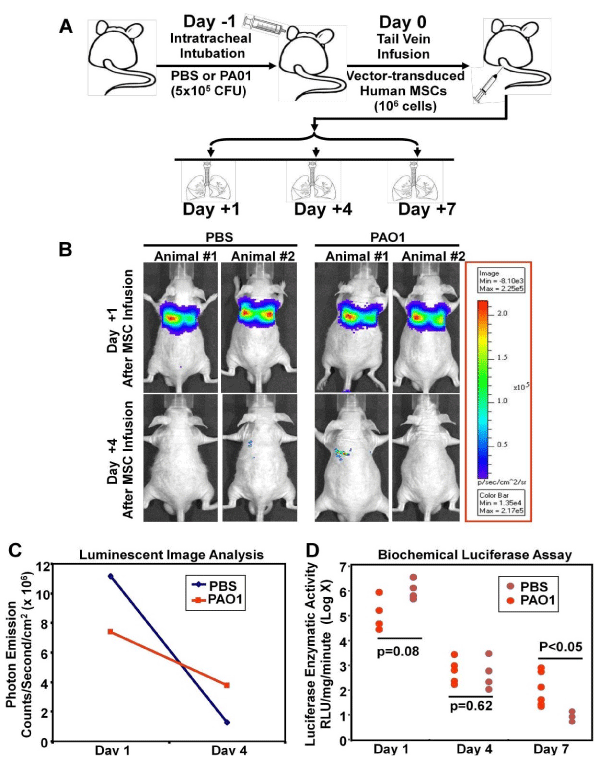
| Figure 4: Lentivector-enhanced expression of FPR in human MSCs facilitates their homing to inflamed lungs. Panel A: Experimental scheme: at Day -1, athymic (nu/nu) mice were intubated intra- tracheally with 50 μl of Pseudomonas aeruginosa suspension solution (5 x 105 CFU) or the same volume of PBS for control. At Day 0, human MSCs (1 x 106), co-transduced with 20 MOI of HIV- CMV-FPR-IRES2-EGFP and HIV-CMV-Luciferase-IRES2-DsRed vectors, were infused into the mice by tail vein injection. At the specified time points (Day +1, +4 and +7), the animals were either subjected to in vivo bioluminescent imaging or sacrificed for measurement of firefly luciferase enzymatic activity in the lung. Panels B & C: Whole-body bioluminescent imaging of MSC localization. Two animals from each group were anesthetized by isoflurane inhalation and injected with 150 mg/kg of luciferin. Bioluminescent signals were detected at 5 minutes after luciferin injection at an integration time of 1 second to 2 minutes using the Xenogen in vivo imaging system. The intensity of bioluminescent emission was pseudo-colored and displayed (B). Photon emission rates in the thorax area were obtained and demonstrated (C). Panel D: Biochemical measurement of firefly luciferase enzymatic activity in the lungs. The animals were sacrificed at Day +1, +4 and +7 post MSC-infusion. The lungs were harvested and homogenized. Luminescence was measured immediately after addition of luciferin in a luminometer and normalized against the protein concentrations determined by BCA analyses. The data were expressed as Relative Luminescent Units (RLU) per milligram protein (mg) per minute. By day 7, significant retention of the MSCs in the lungs challenged with Pseudomonas aeruginosa was detected. |