 |
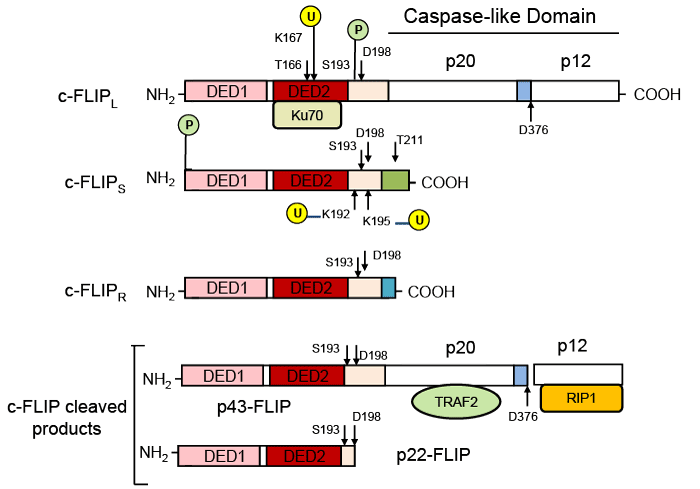
| Figure 2: Structures of c-FLIP variants and cleavage products. c-FLIP isoforms (c-FLIPL, c-FLIPS, and c-FLIPR) contain two death effector domains (DED1 and DED2) at their N termini which are required for DISC recruitment. In addition to two DEDs, c-FLIPL has a significant similarity to caspase 8 and has a large (p20) and a small (p12) caspase-like domain which are catalytically inactive. c-FLIPS and c-FLIPR consist of two DEDs and a small C terminus. c-FLIPL can be cleaved by caspase-8 generating the N-terminal fragment p43-FLIP or p22-FLIP. The phosphorylation (P) sites and ubiquitination (U) sites are indicated [35,37,60]. The p20/p12 regions interact with TRAF2 and RIP1, respectively and Ku70 bind to DED2 [57]. |