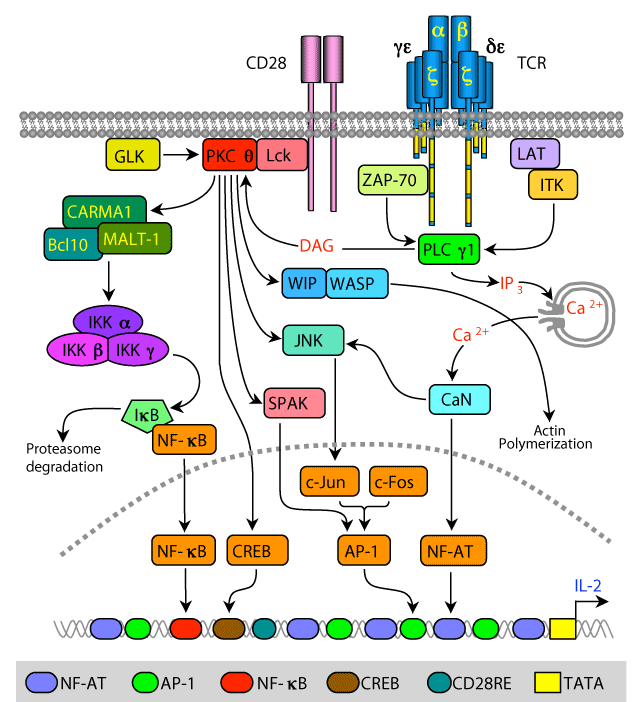
TCR/CD28 engagement triggers the activation of protein tyrosine kinases (PTKs) and phosphorylation of multiple substrates, including the cytoplasmic tail of the CD28. As a result, the phosphorylated CD28 becomes a docking site for the cytoplasmic Lck PTK, which is tethered to the plasma membrane via its constitutive association with the cytoplasmic tail of CD4 or CD8 (not show) and its N-terminal fatty acid residues. Simultaneous activation of phospholipase C γ1 (PLCγ1), which hydrolyzes the membrane phospholipid, phosphatidylinositol 4,5-bisphosphate (PIP2) to diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3), enables PKCθ anchoring to the plasma membrane and the release of Ca2+ ions from intracellular stores, respectively. Colocalization of PKCθ and CD28 in the immunological synapse is regulated by PKCθ interaction with Lck and formation of a trimolecular complex comprising of CD28-Lck-PKCθ.
DAG anchoring of PKCθ to the plasma membrane, in addition to the indirect association of PKCθ with CD28, the phosphorylation of PKCθ by Lck and the germinal center kinase (GCK)-like kinase (GLK), as well as autophosphorylation result in activation of the PKCθ catalytic domain. As a result, PKCθ phosphorylates several substrates, including CARD (caspaseassociated recruitment domain)-containing MAGUK (membrane-associated guanylate kinase) protein 1 (CARMA1), cAMP responsive element binding protein (CREB), STE20/SPS1-related proline/alanine-rich kinase (SPAK), and c-Jun N-terminal kinase (JNK), which are directly or indirectly involved in signaling pathways regulating the interleukin-2 (IL-2 gene) transcription.
Phosphorylation of the scaffold protein, CARMA1, results in a conformational change that allows CARMA1 interaction with B-cell lymphoma/leukemia 10 (Bcl-10) and mucosa-associated lymphoid tissue lymphoma translocation gene 1 (MALT1), forming a trimolecular complex that activates the IκB kinases (IKKs). Phosphorylation of IκB, which detaches from the p65 and p50 subunits of nuclear factor-κB (NF-κB), and is degraded by the S26 proteasome, enables the p65 and p50 heterodimer enter the nucleus and target the IL-2 promoter NF-κB sites.
Phosphorylation and activation of JNK leads to phosphorylation of c-Jun and its dimerization with a newly synthesized c-Fos, forming an active AP-1 transcription factor that enters the nucleus and targets AP-1 binding sites. In addition, PKCθ-mediated phosphorylation and activation of SPAK increases AP-1 activity via an unknown mechanism. PKCθ also phosphorylates the CREB transcription factor and promotes its binding to the CREB site in the IL-2 promoter, although the effect on gene transcription appears to be regulated by the relative activity of phosphorylated CREB and cAMP-responsive element modulator (CREM).
Phosphorylation of the Wiskott-Aldrich Syndrome Protein (WASP)-interacting (WIP) by PKCθ disengages it from WASP and releases WASP from WIP inhibition. This promotes WASP interaction and activation of the Arp2/3 complex that regulate actin polymerization. Calcineurin (CaN), which is activated by Ca2+ ions, dephosphorylates cytoplasmic NFAT proteins, exposing their nuclear localization sequences and inducing their translocation into the nucleus where they bind NFAT sites on the IL-2 gene promoter together with the c-Jun/c-Fos dimers.