 |
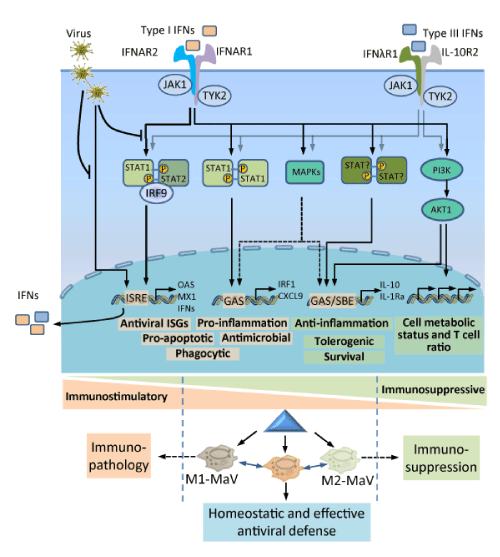
| Figure 2: Ramification of IFN signaling pathways leading to immunostimulatory and immunosuppressive regulation of macrophage polarization. Viral infection of tissue-resident macrophages or nearby cells leads to production of type I and type III IFNs, which are perceived by distinct membrane-bound receptor complexes but stimulate similar signaling pathways in the infected or other proximal macrophages. In addition to the canonical signaling pathway through STAT1/STAT2/IRF9 (also known as the ISGF3) binding to IFN-stimulated response elements (ISREs) in gene promoters, leading to induction of a large number of IFN-stimulated genes (ISGs) and pro-inflammatory responses, both types of IFNs, in particular manifested using type I IFNs, also signal through STAT1 homodimers, which are more commonly associated with the IFNγ-mediated signaling pathway for classical activation (M1) macrophages. Other STAT heterodimers and homodimers (including STAT3-6) may also be activated but lead to production of anti-inflammatory and immunosuppressive IL-10 and IL-1Ra. Other STAT-independent signaling pathways including MAPK- and PI3K-pathways also may be activated, thereby exerting diverse effects in macrophages as well as other immune cells (such effects on T cell, in particular Treg cell ratio), which critically regulate the outcomes of virus-host interaction through, at least in part, the modulation of macrophage polarization [30,31]. |