 |
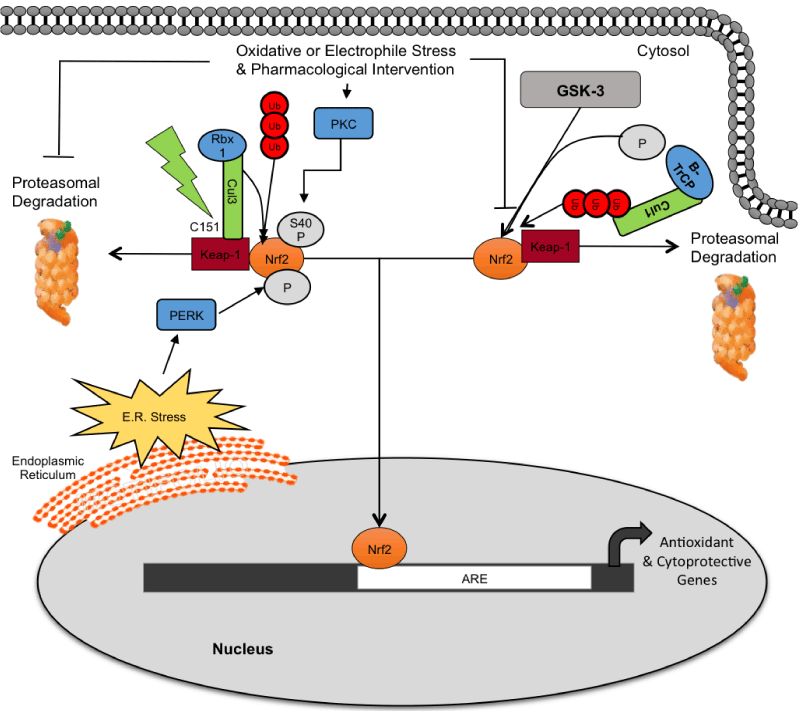
| Figure 2: Cytosolic regulation of Nrf2. Under unstressed conditions, Nrf2 is located in the cytosol where it is associated with a negative regulator Kelch-like ECHassociated protein 1 (Keap1), which is associated with Cul3 and Rbx, forming an E3 ubiquitin ligase complex that actively targets the lysine residues of Nrf2 for ubiquitination. Nrf2 protein is rapidly turned over in a Keap1-dependent manner through Cul3-Rbx1 ubiquitination and proteasomal degradation. When cells are exposed to oxidative stress, electrophiles, or pharmacological agents, Nrf2 escapes Keap1-mediated repression, allowing it to translocate from the cytosol to the nucleus. Cellular stress can disrupt the Nrf2-Keap1 complex directly through directly modifying cysteine residues, the PKC pathway, or via the PERK pathway as a result of ER stress. Degradation of the Nrf2 transcription factors also occurs in a Keap1-indepdent manner by the Glycogen Synthase Kinase 3/β-TrCP axis via a phosphodegron in the Neh6 domain [28-30]. Briefly, GSK3 phosphorylates Ser335 and Ser338 in the DSGIS338 of Nrf2 which increases β-TrCP binding and ubiquitination. |