 |
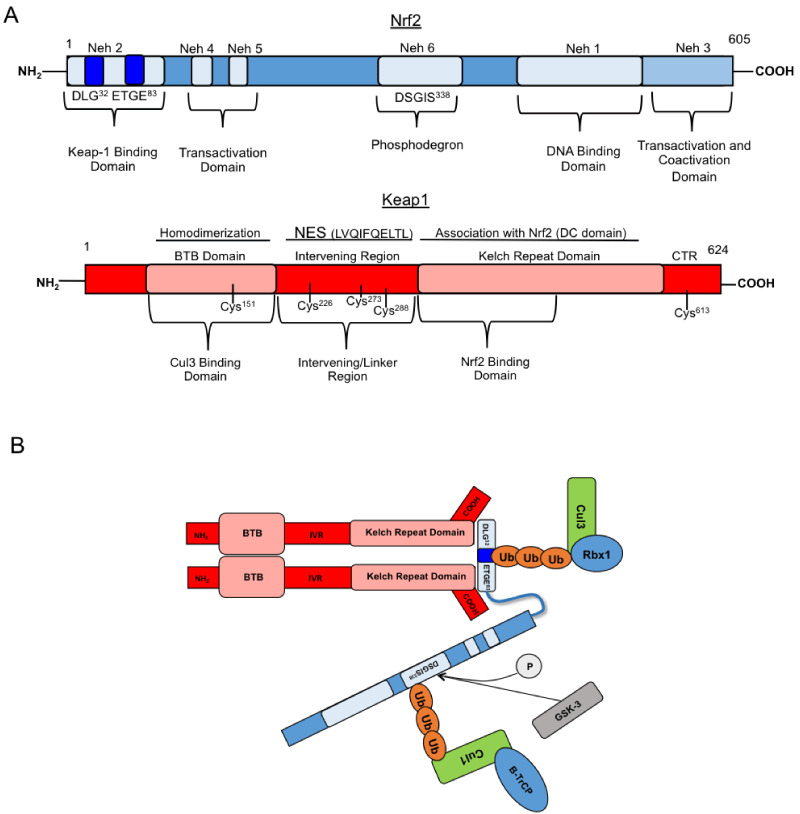
| Figure 1: Structures of Nrf2 and Keap1 and Keap1-mediated ubiquitination and GSK-3/β-TrCP mediated ubiquitination. A) Human Nrf2 contains 589 amino acids and six highly conserved Nrf2-ECH homology (Neh) domains. Neh 1 domain is the DNA-binding domain that interacts with small Maf proteins, HATs, and HDACs. The Neh2 domain contains seven lysine residues for ubiquitin and serves as the binding domain for the Kelch domain of Keap-1. The Neh 3 domain is requisite for transcription through the recruitment of CHD6, a coactivator that contains a helicase and chromodomain. Neh 4 and 5 are transcription transactivation domains that bind to p300/CBP and act synergistically to optimize activation of reporter gene expression. The Neh 6 domain is a serine rich domain that is believed be involved in regulation of degradation and regulation through phosphorylation. Human Keap1 contains 624 amino acids and five domains. The BTB domain functions with the N-terminus to mediate homodimerization and binding of Keap1 to Cul3. The Kelch domain functions with the C-terminus to mediate the binding of Nrf2 to Keap1. B) Degradation of Nrf2 is regulated through the Keap1-Cul3-Rbx E3 ubiqutin ligase complex in the Neh2 domain and by the Glycogen Synthase Kinase 3/β-TrCP axis via a phosphodegron in the Neh6 domain. |