 |
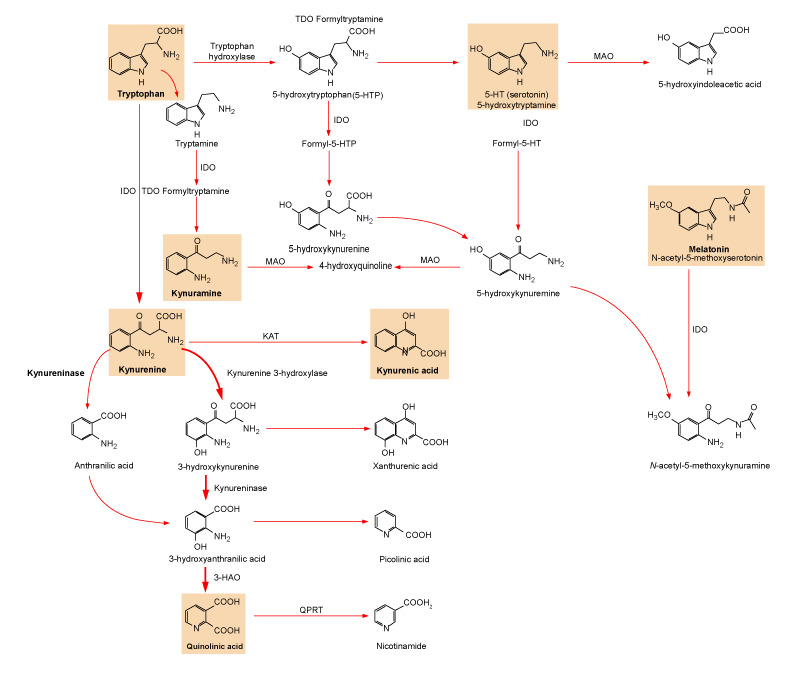
| Figure 1: Various pathways of the essential amino acid tryptophan metabolism. About 99% of the dietary tryptophan is metabolized along the kynurenine pathway (red arrows). Alternative pathways are the conversion of tryptophan to 5-hydroxytryptamine (5-HT) and then to melatonin, or to tryptamine and then to the kynuramines (or kynurenamines). N1-acetyl-5-methoxykynuramine is a metabolite deriving from melatonin by mechanisms involving free radicals, exhibits potent antioxidant properties exceeding those of its direct precursor N1-acetyl-N2-formyl-5-methoxykynuramine, a biogenic amine generated through either an enzymatic or a chemical reaction (free radicals) pathway. 3-HAO, 3-hydroxyanthranilate oxidase; IDO, indoleamine 2,3-dioxygenase; KAT, Kynurenine aminotransferase; MAO, monoamine oxidase; QPRT, quinolinic-acid phosphoribosyl transferase; TDO, tryptophan 2,3-dioxygenase [200]. |