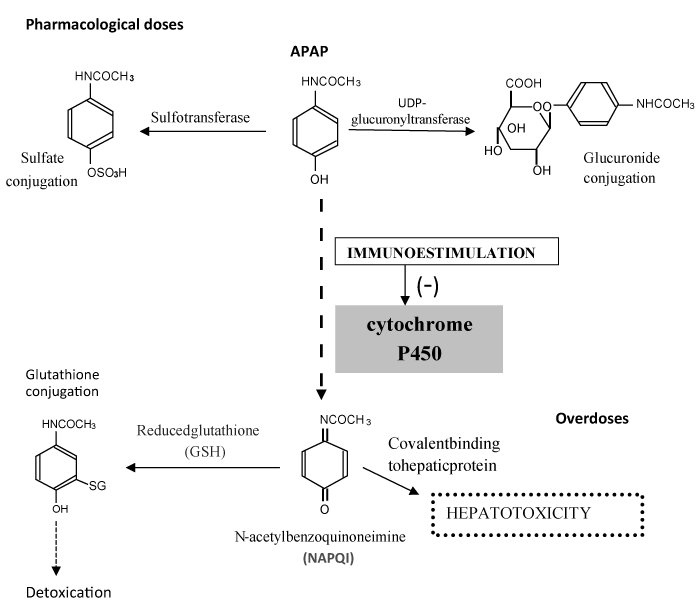
Acetaminophen (APAP) is biotransformed and eliminated as nontoxic sulfate and glucuronic acid conjugates. Cytochrome P450 (CYP) participates in metabolizing a small proportion of APAP at therapeutic doses. The metabolism of APAP by CYP leads to the formation of N-acetylpbenzoquinoneimine (NAPQI), a highly reactive intermediate metabolite, which is normally detoxified by conjugation with reduced glutathione (GSH). After high doses of APAP, the capacity for its removal by hepatic conjugation with glucuronide and sulfate is exceeded, and more of the reactive metabolite NAPQI is formed. Consequently, more NAPQI is conjugated with GSH, and when hepatic GSH is depleted, more NAPQI will bind covalently to cellular macromolecules; this lead to a loss of protein thiol-groups and ultimately to cell necrosis (hepatotoxicity)