Research Article Open Access
Application of Monooxygenases in Dehalogenation, Desulphurization, Denitrification and Hydroxylation of Aromatic Compounds
| Pankaj Kumar Arora1, Alok Srivastava2* and Vijay Pal Singh2 | |
| 1Environmental Biotechnology, Microbial Type Culture Collection and Gene Bank (MTCC), Institute of Microbial Technology, Sector-39A, Chandigarh-160036, India | |
| 2Department of Plant Science, Faculty of Applied Sciences, M.J.P. Rohilkhand University, Bareilly -243006, India | |
| Corresponding Author : | Alok srivastava Department of Plant Science Faculty of Applied Sciences Rohilkhand University, Bareilly-243006, India Tel. +919760821347 E-mail: alok.plsc@rediffmail.com |
| Received October 25, 2010; Accepted November 25, 2010; Published November 27, 2010 | |
| Citation: Arora PK, Srivastava A, Singh VP (2010) Application of Monooxygenases in Dehalogenation, Desulphurization, Denitrification and Hydroxylation of Aromatic Compounds. J Bioremed Biodegrad 1:112. doi: 10.4172/2155-6199.1000112 | |
| Copyright: © 2010 Arora PK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Monooxygenases act as biocatalysts in bioremediation process and synthetic chemistry due to their highly regioselectivity and sterioselectivity on wide range of substrates. They are involved in the process of desulfurization, dehalogenation, denitrification, ammonification, hydroxylation, biotransformation and biodegradation of various aromatic and aliphatic compounds. In the recent years, the practical applications of monooxygenases have been improved using the approaches of directed evolution, meta-genomics and bioinformatics. This review is focused on current applications of monooxygenses especially in biodegradation and biotransformation of aromatic compounds.
| Keywords |
| Monooxygenases; Desulfurization; Dehalogenation; Aromatic compounds; Biodegradation |
| Introduction |
| Aromatic compounds are persistence environmental pollutants that are widely distributed in the biosphere due to anthropogenic activities. These compounds are highly toxic to living beings; therefore, many of them have been listed as priority pollutants by United States Environmental Protection Agency. Microbial degradation has emerged as an effective technology to remove these compounds from environment. Many microbial enzymes such as oxygenases, dehalogenases, reductases, hydroxylases and dehydrogenases are involved in the degradation of these environmental pollutants. Among them, oxygenases are the key enzymes for aerobic biodegradation of aromatic compounds because they are involved in the initial reaction of degradation and also catalyze the ring cleavage of the aromatic compounds which is the essential step for the complete mineralization of these compounds [1]. |
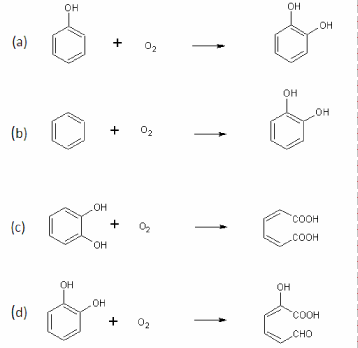
| Oxygenases catalyze insertion of one or two oxygen atoms into the substrates. Two classes of oxygeases have been identified based on number of oxygen atom used in the oxidation: monooxygenases and dioxygenases [1]. Monooxygenases incorporate one atom of the oxygen into the substrate (Figure 1a) whereas dioxygenases add both atoms of the oxygen [1]. |
| Monooxygenases are classified into two subclasses based on which cofactor is present: Flavin dependent monooxygenases and P450 monooxygenases. Flavin dependent monooxygenases contain flavin as prostethic group and require NADP or NADPH as coenzyme [2]. P450 monooxygenases are hame containing oxygenases and found in both eukaryotic and prokaryrotic organisms. Best example of bacterial P450 monooxygenase is CYP102 from Bacillus megaterium BM3 that is able to hydroxylate a variety of alkanes, fatty acids and aromatic compounds [3]. Other types of bacterial monooxygenases are also known that do not contain flavin or hame as a cofactor e.g., pterin-dependent monooxygenases and metal ion-dependent monooxygenases [4]. Majority of monooxygenases require cofactor for their activities, however, some monooxygenases have also been identified and characterized that do not require any cofactor for their activities. These cofactor independent monooxygenases require only molecular oxygen for their activities and utilize the substrate as reducing agent. Examples of these mononoxygenases are tetracenomycin F1 monooxygenase (TcmH) from Streptomyces glauscens [5]; ActVAorf6 monooxygenase from Streptomyces coelicolor A3(2) [6]; quinol monooxygenase (YgiN) from E. Coli [7] and Rv0793 a hypothetical monooxygenase from Mycobacterium tuberculosis [8]. |
| Aromatic dioxygenase are also divided into two subclasses based on mode of action: aromatic ring hydroxylation dioxygenases (ARHDs) and aromatic ring cleavage dioxygenases (ARCDs) [1]. ARHD catalyzes the incorporation of both atoms of oxygen into aromatic ring (Figure 1b) whereas ARCD catalyzes ring cleavage of the aromatic ring typically carrying two or more hydroxyl groups [1]. ARCDs are further divided into two groups based on the ring cleavage: intradiol (IARCD) and extradiol (EARCD) [1]. Intradiol cleaves the aromatic ring between two hydroxyl groups (ortho-cleavage) (Figure 1c), whereas extradiol cleaves the aromatic ring between a hydroxylated carbon and an adjacent non-hydroxylated carbon (meta-cleavage) (Figure 1d). |
| Several publications have been focused on dioxygenases and their role in biodegradation [9-12]. But very few reviews are available that cover the role of the monooxygenases in biodegradation [13]. In this review, we have discussed the current applications of the monooxygenases in biodegradation and biotransformation of aromatic compounds. |
| Monooxygenases and Dehalogenation of Polychlorinated Aromatic Compounds |
| Polychlorinated compounds are widely distributed in the environment and cause serious health problem to humans beings and animals. These compounds are considered recalcitrant due to presence of multiple chlorine atoms at benzene ring. Examples of these compounds are polychlorinated biphenyls (PCB), pentachlorophenol (PCP), dichlorophenol (DCP), trichlorophenol (TCP), dioxin, 2, 4-dichlorophenoxyacetic acid (2,4-D) and dichloropheyltrichloroethane (DDT). |
| Microbial degradation of polychlorinated aromatic compounds initiated with the removal of the chloride atom from a benzene ring. Chloride atom can be removed from aromatic ring in three different ways: hydrolytic, reductive and oxygen dependent dehalogenation [14]. Hydrolytic dehalogenation includes replacement of chlorine atom with hydroxyl group. This hydroxyl group is derived from water. Reductive dehalogenation involves replacement of chlorine atom with hydrogen atom whereas oxygenolytic dehalogenation involves replacement of chlorine atom by hydroxyl group whose oxygen atom is derived from O2. |
| Oxygenolytic dehalogenation is further divided into two classes: dioxygenase type dehalogenation and monooxygenase type dehalogenation. Dioxygenase type dehalogenase adds two atoms of oxygen into the substrate to remove the chlorine atom. They involved in dehalogenation of monochlorinated aromatic compounds such as 4-chlorophenyl acetate and 2-chlorobenzoate [14]. Monooxygenase type dehalogenase adds one atom of oxygen to chlorinated compounds to remove the chlorine atom. Examples of monooxygenase type dehalogenases are pentachlorophenol 4-monooxygenase, chlorophenol 4-monooxygenase and 2, 4, 6-trichlorophenol monooxygenase. These monooxygenases are involved in dehalogenation of polychlorinated aromatic compounds and have been characterized and purified from a variety of microorganisms. |
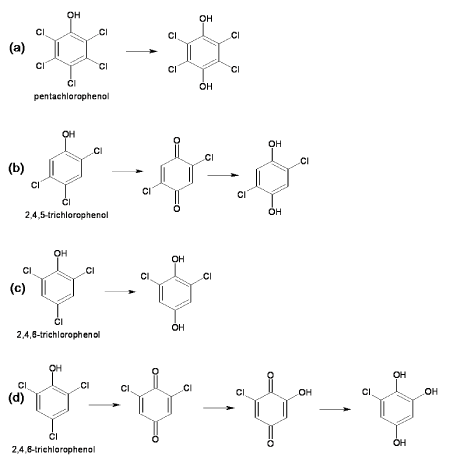
| Pentachlorophenol 4-monooxygenase (EC 1.14.13.50) |
| Pentachlorophenol 4-monooxygenase (PcpB) is a flavin monooxygenase that catalyzes hydroxylation at para position with removal of the chloride ion in the initial step of the microbial degradation of pentachlorophenol (Figure 2a). As a result of hydroxylation, pentachlorophenol converted to tetrahydroquinone [15]. PcpB also catalyzes reaction on various substituted aromatic compounds with release of nitro, amino or cyano group from para position [16]. PcpB is encoded by the pcpB gene that has been identified in a variety of PCP degrading bacteria [17,18]. Examples of PCP degrading bacteria are Sphingonium, Sphingomonads and Pseudomonas. The pcpB gene was identified in the four strains of Sphingonium chlorophenolica (ATCC 39723, RP-2, SR-3 and ATCC 33790) in which three strains (ATCC 39723, RP-2, SR-3) had identical pcpB gene sequence [19,20] whereas the sequence of the pcpB gene of strain ATCC 33790 was differed by 10% from rest of three strains [20]. The pcpB gene sequence of Sphinogomonads strain UG-30 showed 90% sequence similarity with that of Sphingonium chlorophenolica ATCC 39723 [21-23]. The homologus of pcpB gene has been identified in polychlorinated degrading bacterium Novosphingonium sp. strain MT1 [24] as well as in two non-PCP degrading β;- and γ- proteobacterial strains isolated from soil samples from a PCP-contaminated wood treatment site [18]. |
| Pentachlorophenol 4-monoxygenase (PcpB) has been purified and characterized from Sphingomonas chlorophenolica ATCC 39723. PcpB was comprised of 538 amino acids with molecular weight of 39 kD. PcpB was synthesized in cytoplasm and then translocated to periplasm via inner memberane [25]. The crystal structure of PcpB is yet not solved. However, model of 3D structure of PcpB has been proposed on the basis of homology modelling [26]. The active site residue has been determined from proposed 3D structure and site directed mutagenesis was carried out to change the active site residue [26]. Mutant thus obtained produced mutant proteins that purified by affinity chromatography. The finding of mutation study supported the validity of proposed 3D structure based on homology modelling [26] . On the basis of experiment of site directed mutagenesis of active site of PcpB, Nakamura et al. (2004) concluded that Phe 85, Try 216 and Arg 235 are for enzyme activity and Tyr 397 and Phe 87 stablize the structure of the protein [26]. |
| Chlorophenol 4-monooxygenase (EC 1.14.13.-) |
| This enzyme is involved in the degradation pathway of 2, 4, 5-trichlorophenoxy acetic acid by Burkholderia cepacia AC1120 [27]. This enzyme catalyzes conversion of 2,4,5-trichlorophenol (2,4,5- TCP) to 2,5-dichloro-p-benzoquinone, which is chemically reduced to 2,5-dichloro-p-hydroquinone (2,5-DiCHQ) (Figure 2b) [28]. The gene encoding chlorophenol monooxygenase (tftd) has been identified, sequenced and cloned from Burkholderia cepacia AC1100 and transformed into E. coli for overexpression [27-30]. The molecular weight of the purified protein was 58 kD [28]. This protein utilizes O2, FAD and NADH for catalyzing the reaction but does not utilize riboflavin and NADPH [28]. |
| 2, 4, 6-Trichlorophenol Monooxygenase (EC 1.14.13.-) |
| This enzyme was first reported in Azotobacter sp. GP1 and catalyzes conversion of 2,4,6 trichlorophenol to 2,6-dichlorohydroquinone with release of chloride ion (Figure 2c) [31]. TCP monooxygenase from Azotobacter was a homotetrameric protein with molecular weight of 240 kD. Another 2,4,6-trichlorophenol (2,4,6-TCP) 4-monooxygenase was found in Ralstonia eutropha JMP134 that catalyzes sequential dechlorinations of 2,4,6-TCP to 6-chlorohydroxyquinol [32]. This monooxygenase oxidize 2, 4, 6-TCP to 2, 6-dichloroquinone that further hydrolyzed to 2-chlorohydroxyquinone by the same enzyme (Figure 2d). 2-Chlorohydroxyquinone was then reduced by ascorbate and NADH to 6-chlorohydroxyquinol [32]. |
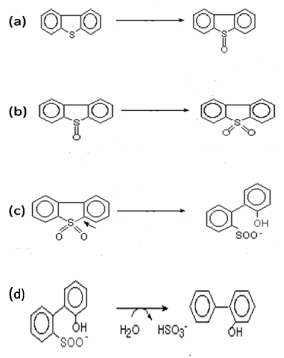
| Monooxygenases Involved in Biodesulfurization of Dibenzothiophene |
| Sulphur containing organic compounds such as dibenzothiophene (DBT) are present in the fossil fuels. On combustion of these fossil fuels, sulphur dioxide is released into the environment and causes air pollution. The complete removal of sulphur from these compounds is not possible by conventional physical and chemical methods. Biodesulfurization, therefore, is a process that completely removes sulphur from these organic compounds. Biodesulfurization of DBT is well characterized in Rhodococcus erythropolis IGTS8 [33]. Two flavin dependent monooxygenases, DBT monooxygenase (DszC) and DBT sulfone monooxygenases (DszA) are involved in the initial steps of the biodesulfurization. DBT monooxygenase (EC 1.14.13.-) converts DBT to DBT sulphoxide which is further converted to DBT sulphone by same enzyme (Figure 3a and 3b). DBT sulfone monooxygenase (EC 1.14.13.-) converts DBT sulfone to 2’-hydroxybiphenyl 2-sulfinate (HBPS) (Figure 3c). Both of the monooxygenases require another enzyme flavin reductase (DszD) for their activities. One more enzyme HBPS desulfinase (DszB) is also involved in the final step of biodesulfurization and converts HBPS to hydroxybiphenyl (HBP) and sulphate (Figure 3d). In R. erythropolis, enzymes DszA, DszB and DszC are encoded by plasmid located dsz operon and another enzyme DszD is considered as genome encoded [34,35]. The desulfurization genes was conserved among Rhodococcus species [36]. Several strains of R. erythropolis, i.e., SY1, D-1, Ni-36, Ni-43, and QIA-22 exhibited same DBT-desulfurizing reaction as reported in Rhodococcus erythropolis IGTS8 [37-40]. |
| Although the biochemistry, genetics and physiology of desulphurization have been extensively studied in Rhodococcus sp., the desulfurizing genes (dszABC) have also been identified in other mesophilc bacteria [41-43] as well as thermophilc bacteria. The sequences of the dszABC genes of a thermophilic bacterium Mycobacterium phlei that carry out biodesulfurization at range of 20- 50°C, was found to be identical to that of Rhodococcus erythropolis IGTS8 [44-46]. The DszA and DszC from the thermophilic bacterium Paenibacillus sp. strain A11-2 have been purified and characterized [47]. DszA is dimeric protein with molecular weight of 120 kD and subunit molecular weight of 48 kD whereas DszC was tetrameric protein with molecular weight of 200 kD and subunit mass 43 kD. The optimum temperature for activity of DszA and DszC was determined 44°C and 65°C respectively. |
| Recombinant bacteria have also been created to enhance the activity of biodesulfurization [48,49]. The recombinant strain desulfurizes DBT more efficiently than the native one, and also provides new alternative for the development of a commercial desulfurization process [50]. A genetically modified organism Pseudomonas putida CECT 5279 carrying dszABC genes from Rhodococcus erythropolis IGTS8 and a flavin oxidoreductase (hpac) from E.coli was constructed for biodesulfurization process [51]. This recombinant strain produced maximum amount of DszB enzyme at the early exponential phase that rapidly decreased at the middle exponential phase, hampering an efficient transformation of HBPS into HBP. To overcome this problem, a model two-step BDS resting cell process using P. putida cells from the late and early exponential growth phases was designed to significantly increase biodesulfurization. In the first step, the cells of late exponential phase (10 h grown cells) of P. putida CECT 5279 showing the maximum activities of DszA and DszC monooxygenases, were transformed all DBT into 50% mixture of HBPS and HBP [51]. In the second step, the cells of late exponential phase have removed and the early exponential phase cells (5 h grown cells) were added. After addition remaining HBPS was efficiently converted to HBP [51]. |
| Another recombinant bacteria has been design by transferring the pVLT31 vector harboring flavin-oxidoreductase gene (dszD) into the recombinant P. aeruginosa ATCC 9027 which contained dszABC gene in its chromosome stably [52]. This recombinant bacteria showed the enhanced activity of biodesulfurization when all four genes co expressed. The biodesulfurization efficiency has also been significantly improved using the approach of directed evolution. Arensdorf et al. (2002) presented an example of directed evolution of biodesulfurization using chemostat approach [53]. Rhodococcus erythropolis IGTS8 has not ability to utilize octyl sulphide and 5-methylbenzothiophene (5-MBT) as sole sulphur sources. Arensdorf et al. (2002) selected gain-of-function mutants of strain IGTS8 using sulphur limited chemostat [53]. These mutants were grown with octyl sulphide and 5-MBT as sole sulphur sources. The ability of mutant to grow on 5-MBT as sole sulphur source was due to a transversion (guanine to thymine) in dszC codon 261 [53]. |
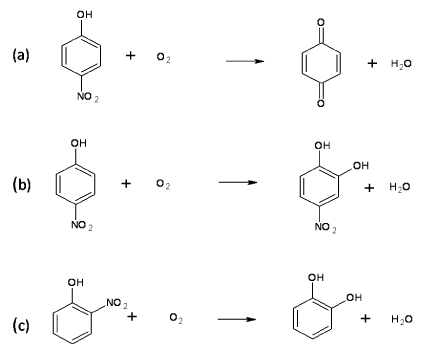
| Monooxygenases and Denitrification of Nitro Aromatic Compounds |
| Nitroaromatic compounds are serious environment pollutants that are used in the synthesis of pesticides, drugs, dyes and explosives [54]. Nitro phenols are the most common examples of nitroaromatic compounds. The toxicity of these compounds is mainly due to the presence of nitro group in the aromatic ring. The aerobic degradation of these compounds generally initiate with removal of the nitro group by oxygenase activity [55]. Examples of monooxygenases involved in denitrification of nitroaromatic compounds are 4-nitrophenol 4-monooxygenase, 4-nitrophenol 2-monooxygenase and 2-nitrophenol 2-monooxygenase. 4-Nitrophenol 4-monooxygenase (EC 1.14.13.-) has been purified and characterized from Pseudomonas sp. strain WBC-3 [56]. This enzyme is a single component and flavin adenine dinucelotide monooxygenase that catalyzes conversion of p-nitrophenol to p-benzoquinone in the presence of NADPH (Figure 4a). This enzyme also catalyzes NADPH dependent conversion of nitrocatechol to 1, 2, 4-benzenetriol [57]. Another denitrifying 4-nitrophenol 2-monooxygenase (EC 1.14.13.29) has been purified and characterized from Rhodococcus sp. strain PN1 [58]. This enzyme is a two-component flavin adenine dinucelotide monooxygenase and converts p-nitrophenol to 4-nitrocatechol in the presence of NADH (Figure 4b) whereas NADPH dependent 2-nitrophenol- 2-monooxygenase (EC 1.14.13.31) catalyzes the conversion of 2-nitrophenol to catechol (Figure 4c) [55]. |
| Monooxygenases and hydroxylation of aromatic compounds |
| Several monooxygenases have been identified and characterized that involved in the biodegradation of various aromatic compounds and have been listed in the (table 1). A few monooxygenases acting on aromatic compounds have been used as biocatalysis for synthesis of the pharmaceuticals compounds. Examples of these monooxygenases are styrene monooxygenase, hydroxybiphenyl 3-monooxygenase and phenylacetone monooxygenase. Styrene monooxygenase (EC 1.14.13.-) from Pseudomonas sp. VLB 120 has ability to convert styrene to s-styrene oxide with an enantiomeric excess of greater than 99% [81]. Styrene monooxygenase is a two component enzyme: StyA (oxygenase) and StyB (reductase). A recombinant E. Coli expressing StyA and StyB has been used as whole cell biocatalyst for a broad substrate spectrum that gives access to various chiral acryloxides [82]. Another commercially important enzyme, 2-hydroxybiphenyl 3-monooxygenase (HbpA, EC 1.14.13.44) from Pseudomonas azelaica HBP1 catalyzes ortho-hydroxylation of 2-hydroxybiphenyl to 2, 3-dihydroxybiphenyl and also catalyzes the conversion of different 2-substituted phenols to 3-substituted catechols that are of interests of pharmaceuticals industry [83,84] . The substrate reactivity of 2-hydroxybiphenyl 3-monooxygenase (HbpA) have been improved by directed evolution [84]. A variant of recombinant E. coli JM101 expressing HbpA was used for the production of 3-tert-butylcatechol, a compound of pharmaceutical interest [86]. |
| Phenylacetone monooxygenase (PAMO, EC 1.14.13.92) is a FADcontaining Baeyer-Villiger monooxygenase (BVMO). This enzyme was discovered from Thermobifida fusca that is a moderately thermophilic soil bacterium and grows at 55°C. PAMO oxidizes phenylacetone, aromatic and aliphatic ketones, aromatic sulphides and sulphoxides and organoborn compounds [87]. This enzyme has thermostability and tolerance towards organic solvents [88], therefore, this enzyme is suitable for various industrial applications. |
| Current ways to imporve the efficiency of monooxygenases |
| Metagenomic approach: Microbial diversity is a rich source of novel enzymes including monooxygenases. To date, only 1% microorganisms could be cultured in the laboratory conditions and monooxygenases associated with these culturable microbes have been identified and characterized. Most potential monooxygenases remain untapped because vast majority of bacteria are uncluturable. In such a case, metagenomics may be powerful tool to explore novel monooxygenases from entire community of microbes. The metagenomic approach involves (i) the isolation and purification of DNA from an environmental sample, (ii) cloning of DNA fragments into suitable vectors, (iii) the transformation of host cells with construct and (iv) functional and sequence based screening of constructed clones. Numerous novel biocatalysts have been isolated from various metagenomic libraries. A novel styrene monooxygenase (SmoA) was discovered by the screening of a metagenomic library from loam soil [89]. SmoA is highly enantioselective, since it is able to convert aromatic alkenes into the (S)-epoxides with an excellent enantiomeric excess. Recently, two flavin monooxygenases catalyzing the oxidation of indole to a mixture of indigo and indirubin pigments have been identified from an effluent treatment plant sludge metagenomic library. A new self-sufficient CYP, SYK181was identified during sequence-based screening of a metagenomic library [91]. SYK181 showed significant hydroxylase activity towards naphthalene and phenanthrene as well as towards fatty acids [91]. Therefore, it is very useful for the biodegradation of organic chemicals. |
| Directed evolution: In the recent years, directed evolution is playing a major role for engineering of the proteins for bio catalytic applications. Directed evolution is based on the Darwinian principle of evolution and involves (i) random mutagenesis to generate the diversity and (ii) the subsequent selection of the improved variants by screening. The efficiency of several monooxygenases has been significantly improved by directed evolution. For example, CYP102 from Bacillus megaterium BM3 has been turned by directed evolution into an enzyme that hydrolyze aromatic compounds like 2,6-dichlorophenol and 2-benzyloxyphenol which cannot be hydrolyzed by wild type enzyme [92]. Furthermore, a triple mutant of CYP102A1 has converted β-ionone into flavorant (R)-4-hydroxy-β- ionone in a regioselective manner and exhibited 80-fold higher activity compared to the wild type enzyme [93] . Another example of directed evolution is cyclohexanone monooxygenase (CHMO; EC 1.14.13.22) from Acinetobacter sp. NCIMB 9871. This enzyme act as a biocatalyst in the Baeyer–Villiger (BV) reaction of 4-hydroxycyclohexanone and other 4-substituted cyclohexanone derivatives [94]. It has been used for commercial production of bicyclic lactone, sulfoxides, thiosulfinates and cyclic sulphates [95-97]. The range of the substrates and enantioselectivity of this enzyme have been increased by directed evolution [98,99]. Reetz et al. (2004) demonstrated that some CHMO mutants showed improved enantioselectivity, preferring the (R)-enantiomer (49-54% ee vs 9% ee for the wild type enzyme) in the oxidation of prochiral 4-hydroxycyclohexanone whereas other variants favored (S)-enantiomer (79% ee). |
| Bioinformatic approach: Database providing information about the biochemistry and genetics of monooxygenases are valuable tools of the computational biology. Currently, two databases namely UMBBD (University of Minnesota Biocatalysis/Biodegradation Database) [100,101] and OXDBase (A Database of Biodegradative Oxygenases) [1] are providing the information about all monooxygenases involved in biodegradation. The characteristics feature of these databases is to provide information of the genes and three dimensional structures of the monooxygenases which can help in site directed mutagenesis of the monooxygenases to improve their catalytic properties [1]. The entries of the monooxygenases in OxDBase as well as UM-BBD are linked to various existing databases which provide to users the detailed information of monooxygenases [1]. Since monooxygenases catalyzed biotransformations of the toxic xenobiotic compounds help in reducing the toxicity of the xenobiotics, therefore, the detailed information of these monooxygenases increase our understanding of biodegradation process [1]. |
| Homology modelling is a tool of bioinformatics that use to generate reasonable models of protein structures. The determination of 3D structure of monooxygenase by homology modelling is a helpful to design the experiment of site directed mutagenesis in active site of that protein to improve the efficiency of the monooxygenase. The three dimensional structure of tolune 4–monooxygenase determined by homology modelling has helped in altering the activity of this enzyme by active site engineering for the synthesis of 3-methoxycatechol, methoxyhydroquinone and methylhydroquinone [102]. |
| Conclusion |
| Monooxygenases are multifunctional enzymes and involved in biodesulfurization, dehalogenation, denitrification and hydroxylation of various aromatic compounds. The applications of monooxygenases have been improved using the recent molecular and bioinformatic approaches. |
References
|
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 19209
- [From(publication date):
December-2010 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 14119
- PDF downloads : 5090
