Commentary Open Access
Androgen Receptor and Hepatocellular Carcinoma
Tatsuo Kanda1*, Osamu Yokosuka1 and Masao Omata2,3
1Department of Gastroenterology and Nephrology, Chiba University, Graduate School of Medicine, 1-8-1 Inohana, Chuo-ku, Chiba 260-8670, Japan
2Yamanashi Hospitals (Central and Kita) Organization, 1-1-1 Fujimi, Kofu-shi, Yamanashi (400-8506), Japan
3University of Tokyo, 7-3-1, Hongo, Bunkyo-ku, Tokyo (113-8655), Japan
- *Corresponding Author:
- Tatsuo Kanda
Associate Professor, Department of Gastroenterology and Nephrology
Chiba University, Graduate School of Medicine
1-8-1 Inohana, Chuo-ku, Chiba 260-8670, Japan
Tel: +81-43-226 2086
Fax: +81-432262088
E-mail: kanda2t@yahoo.co.jp
Received date: April 22, 2013; Accepted date: June 06, 2013; Published date: June 08, 2013
Citation: Kanda T, Yokosuka O, Omata M (2013) Androgen Receptor and Hepatocellular Carcinoma. J Gastroint Dig Syst S12:012. doi: 10.4172/2161-069X.S12-012
Copyright: © 2013 Kanda T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
The androgen receptor (AR) exists in normal liver as well as in human hepatocellular carcinoma (HCC) tissues. Recent studies revealed that AR plays an important role in hepatitis B viral as well as hepatitis C viral hepatocarcinogenesis. The targeting of AR might be developed as a new therapeutic option against HCC. This article provides information regarding the association between AR and HCC.
Keywords
Androgen receptor; Foxa; HBV; HCV; STAT3; VEGF
Abbreviations
HBV: Hepatitis B Virus; HCV: Hepatitis C Virus; HCC: Hepatocellular Carcinoma; AR: Androgen Receptor; DBD: DNA-Binding Domain; ARE: Androgen Response Element; LBD: Ligand-Binding Domain; VEGF: Vascular Endothelial Growth Factor; STAT3: Signal Transducer and Activator of Transcription-3; Foxa: Vertebrae Forkhead Box A
Introduction
Hepatitis B virus (HBV) and hepatitis C virus (HCV) are the most commonly implicated risk factors of hepatocellular carcinoma (HCC) [1-3]. Irrespective of its cause, gender differences also exist in its prevalence [4].
Androgen receptor (AR), belonging to nuclear receptors, is putatively involved in the development of cancers such as prostate cancer, HCC and pancreatic cancer [5-7]. Androgen as a ligand interacts with AR, leading to a suggested link between transcriptional control and physiology. AR is characterized by a central DNA-binding domain (DBD), which targets AR to specific DNA sequences known as androgen response elements (AREs). The C-terminal half of the receptor encompasses the ligand-binding domain (LBD), which possesses the essential property of androgen recognition and ensures both specificity and selectivity of the physical response: LBD can be thought of as a molecular switch that, upon binding with ligand, shifts the receptor to a transcriptionally active state [8-10].
ARE is present in regulatory elements on target genes such as vascular endothelial growth factor (VEGF) and transforming growth factor beta-1, and regulates the growth and proliferation of hepatocytes [11,12]. Sorafenib, a molecular-targeted agent that inhibits tumor cell proliferation and angiogenesis by inhibiting Raf serine-threonine kinase (MAPKK kinase, MAPKKK), and VEGF, platelet-derived growth factor beta (PDGF), fms-related tyrosine kinase 3 (FLT3), and v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog (C-Kit) receptor tyrosine kinase, was approved for treatment of advanced HCC in Europe, USA, Japan and other countries [13,14]. A selective transforming growth factor beta-1 receptor inhibits phosphorylation of the beta 1 integrin intracytoplasmic tail, blocking invasion of HCC cells [15].
Nagasue et al. reported that HCC had a significantly higher concentration of androgen receptors than did the surrounding liver tissue [6]. There are several reports concerning the expression of AR mRNA and AR protein in tumor and in non-tumor parts of liver, suggesting that AR plays an important role in hepatocarcinogenesis [6,16-35].
Androgen Receptor-Mediating Signaling in HCVrelated Hepatocarcinogenesis
HCV core protein, which has a size of ~20 kDa, is one of the structural proteins. It has been reported that HCV core protein is involved in hepatocarcinogenesis [36-42]. Expression of HCV core protein as well as HCV full-length protein leads to activation of the AR signaling pathway in the presence of androgen [43]. Cell culture-grown HCV infection in human hepatocytes also augments AR-mediated signaling in the presence of androgen [43,44]. These results showed that HCV core protein might cross-talk with the AR signaling pathway for promotion of carcinogenesis.
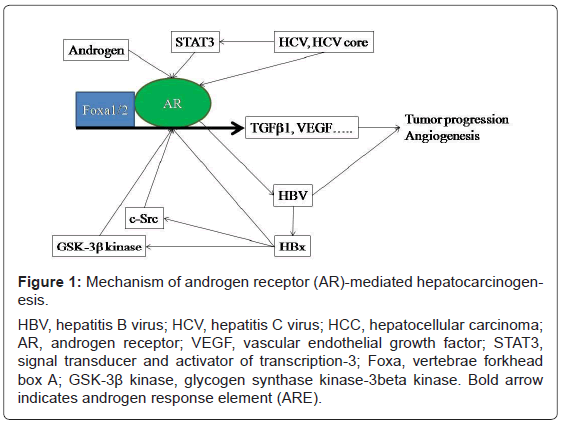
Figure 1: Mechanism of androgen receptor (AR)-mediated hepatocarcinogenesis.
HBV, hepatitis B virus; HCV, hepatitis C virus; HCC, hepatocellular carcinoma;
AR, androgen receptor; VEGF, vascular endothelial growth factor; STAT3,
signal transducer and activator of transcription-3; Foxa, vertebrae forkhead
box A; GSK-3ß kinase, glycogen synthase kinase-3beta kinase. Bold arrow
indicates androgen response element (ARE).
Signal transducer and activator of transcription-3 (STAT3) is often constitutively phosphorylated and activated in human cancers and in transformed cell lines and is implicated in tumorigenesis [45]. HCV core increased STAT3 phosphorylation both at Ser-727 and at Tyr-705, which in turn activates AR [43]. HCV infection also enhances VEGF, one of the target genes of AR [11], and induces in vitro angiogenesis in the presence of androgen [43]. HCV core protein could be an activator of AR in the male-dominant disease HCC.
Androgen Receptor-Mediating Signaling in HBVrelated Hepatocarcinogenesis
The male predominance in HBV-related HCC is significantly high, with a ratio of 5-7:1 [4]. HBx is the only HBV nonstructural gene that functions as a multifunctional regulator modulating gene transcription, cell responses to genotoxic stress, protein degradation, apoptosis, and several signaling pathways [46-48]. HBx, which has a size of ~17 kDa, can physically bind to AR [49]. HBx-mediated enhancement of AR activity is androgen-dependent and could be mediated through indirect mechanism involving calcium and c-Src signaling pathways [50]. HBx enhances the AR transcriptional activity through two kinases: c-Src and glycogen synthase kinase-3beta kinase [51]. AR promotes HBV-induced hepatocarcinogenesis through modulation of HBV RNA transcription [52]. The androgen pathway can increase the transcription of HBV through direct binding to the ARE sites in HBV enhancer I, leading to a higher HBV titer in male carriers and increased risk of HCC [53]. These facts together suggest that the targeting of AR might be developed as a new therapeutic strategy against HBV-related HCC [54].
Ma et al. [54] reported that mice lacking hepatic AR developed later and less HCC than their wild-type littermates with comparable serum testosterone in both males and females when using the Cre-Lox conditional knockout mouse model injected with carcinogen and that AR may promote hepatocarcinogenesis via increased cellular oxidative stress and DNA damage, as well as by suppression of p53-mediated DNA damage sensing/repairing system and cell apoptosis. Recently, it has been shown that vertebrae forkhead box A (Foxa) factors and their targets estrogen receptor (ERα) and/or AR play an important role in the sexual dimorphism of HCC [55]. In conclusion, AR as well as ERα [56] could be involved in human hepatocarcinogenesis. Targeting AR might be developed as new molecular-target therapy against not only HBV-related but also HCV-related HCC.
References
- Hiotis SP, Rahbari NN, Villanueva GA, Klegar E, Luan W, et al. (2012) Hepatitis B vs. hepatitis C infection on viral hepatitis-associated hepatocellular carcinoma. BMC Gastroenterol 12: 64.
- Beasley RP, Hwang LY, Lin CC, Chien CS (1981) Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet 2: 1129-1133.
- Saito I, Miyamura T, Ohbayashi A, Harada H, Katayama T, et al. (1990) Hepatitis C virus infection is associated with the development of hepatocellular carcinoma. Proc Natl Acad Sci U S A 87: 6547-6549.
- Shiratori Y, Shiina S, Imamura M, Kato N, Kanai F, et al. (1995) Characteristic difference of hepatocellular carcinoma between hepatitis B- and C- viral infection in Japan. Hepatology 22: 1027-1033.
- Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, et al. (2004) Molecular determinants of resistance to antiandrogen therapy. Nat Med 10: 33-39.
- Nagasue N, Ito A, Yukaya H, Ogawa Y (1985) Androgen receptors in hepatocellular carcinoma and surrounding parenchyma. Gastroenterology 89: 643-647.
- Okitsu K, Kanda T, Imazeki F, Yonemitsu Y, Ray RB, et al. (2010) Involvement of interleukin-6 and androgen receptor signaling in pancreatic cancer. Genes Cancer 1: 859-867.
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, et al. (1995) The nuclear receptor superfamily: the second decade. Cell 83: 835-839.
- Aranda A, Pascual A (2001) Nuclear hormone receptors and gene expression. Physiol Rev 81: 1269-1304.
- Wu S, Kanda T, Imazeki F, Nakamoto S, Shirasawa H, et al. (2011) Nuclear receptor mRNA expression by HBV in human hepatoblastoma cell lines. Cancer Lett 312: 33-42.
- Bolton EC, So AY, Chaivorapol C, Haqq CM, Li H, et al. (2007) Cell- and gene-specific regulation of primary target genes by the androgen receptor. Genes Dev 21: 2005-2017.
- Yoon G, Kim JY, Choi YK, Won YS, Lim IK (2006) Direct activation of TGF-beta1 transcription by androgen and androgen receptor complex in Huh7 human hepatoma cells and its tumor in nude mice. J Cell Biochem 97: 393-411.
- Kudo M, Ueshima K (2010) Positioning of a molecular-targeted agent, sorafenib, in the treatment algorithm for hepatocellular carcinoma and implication of many complete remission cases in Japan. Oncology 78 Suppl 1: 154-166.
- Shen YC, Hsu C, Cheng AL (2010) Molecular targeted therapy for advanced hepatocellular carcinoma: current status and future perspectives. J Gastroenterol 45: 794-807.
- Kanda T, Imazeki F, Kanai F, Tada M, Yokosuka O, et al. (2012) Signaling pathways involved in molecular carcinogenesis of hepatocellular carcinoma. Molecular Aspects of Hepatocellular Carcinoma. (Qiao L, Yan X, Li Y, George J ed), Bentham Science. 39-55.
- Nagasue N, Kohno H, Chang YC, Hayashi T, Utsumi Y, et al. (1989) Androgen and estrogen receptors in hepatocellular carcinoma and the surrounding liver in women. Cancer 63: 112-116.
- Negro F, Papotti M, Pacchioni D, Galimi F, Bonino F, et al. (1994) Detection of human androgen receptor mRNA in hepatocellular carcinoma by in situ hybridisation. Liver 14: 213-219.
- Iqbal MJ, Wilkinson ML, Johnson PJ, Williams R (1983) Sex steroid receptor proteins in foetal, adult and malignant human liver tissue. Br J Cancer 48: 791-796.
- Wong LY, Chan SH, Oon CJ, Rauff A (1984) Immunocytochemical localization of testosterone in human hepatocellular carcinoma. Histochem J 16: 687-692.
- Wilkinson ML, Iqbal MJ, Williams R (1985) Characterisation of high affinity binding sites of androgens in primary hepatocellular carcinoma. Clin Chim Acta 152: 105-113.
- Ohnishi S, Murakami T, Moriyama T, Mitamura K, Imawari M (1986) Androgen and estrogen receptors in hepatocellular carcinoma and in the surrounding noncancerous liver tissue. Hepatology 6: 440-443.
- Bannister P, Meystre CM, Losowsky MS (1988) Androgen receptor concentrations in needle biopsy specimens of human liver. Liver 8: 28-31.
- Nagasue N, Chang YC, Hayashi T, Galizia G, Kohno H, et al. (1989) Androgen receptor in hepatocellular carcinoma as a prognostic factor after hepatic resection. Ann Surg 209: 424-427.
- Nagasue N, Kohno H, Chang YC, Yamanoi A, Nakamura T, et al. (1990) Clinicopathologic comparisons between estrogen receptor-positive and -negative hepatocellular carcinomas. Ann Surg 212: 150-154.
- Nagasue N, Kohno H, Chang Y, Hayashi T, Nakamura T (1990) Specificity of androgen receptors of hepatocellular carcinoma and liver in humans. Hepatogastroenterology 37: 474-479.
- Nagasue N, Kohno H, Yamanoi A, Kimoto T, Chang YC, et al. (1991) Progesterone receptor in hepatocellular carcinoma. Correlation with androgen and estrogen receptors. Cancer 67: 2501-2505.
- Eagon PK, Francavilla A, DiLeo A, Elm MS, Gennari L, et al. (1991) Quantitation of estrogen and androgen receptors in hepatocellular carcinoma and adjacent normal human liver. Dig Dis Sci 36: 1303-1308.
- Nagasue N, Yamanoi A, Kohno H, Kimoto T, Chang Y, et al. (1992) Androgen receptor in cirrhotic liver, adenomatous hyperplastic nodule and hepatocellular carcinoma in the human. Hepatogastroenterology 39: 455-460.
- Boix L, Bruix J, Castells A, Fuster J, Bru C, et al. (1993) Sex hormone receptors in hepatocellular carcinoma. Is there a rationale for hormonal treatment? J Hepatol 17: 187-191.
- Boix L, Castells A, Bruix J, Solé M, Brú C, et al. (1995) Androgen receptors in hepatocellular carcinoma and surrounding liver: relationship with tumor size and recurrence rate after surgical resection. J Hepatol 22: 616-622.
- Zhang X, He L, Lu Y, Liu M, Huang X (1998) Androgen receptor in primary hepatocellular carcinoma and its clinical significance. Chin Med J (Engl) 111: 1083-1086.
- Tavian D, De Petro G, Pitozzi A, Portolani N, Giulini SM, et al. (2002) Androgen receptor mRNA under-expression in poorly differentiated human hepatocellular carcinoma. Histol Histopathol 17: 1113-1119.
- Wang AG, Lee KY, Kim SY, Choi JY, Lee KH, et al. (2006) The expression of estrogen receptors in hepatocellular carcinoma in Korean patients. Yonsei Med J 47: 811-816.
- Vizoso FJ, Rodriguez M, Altadill A, González-Diéguez ML, Linares A, et al. (2007) Liver expression of steroid hormones and Apolipoprotein D receptors in hepatocellular carcinoma. World J Gastroenterol 13: 3221-3227.
- Kalra M, Mayes J, Assefa S, Kaul AK, Kaul R (2008) Role of sex steroid receptors in pathobiology of hepatocellular carcinoma. World J Gastroenterol 14: 5945-5961.
- Ray RB, Lagging LM, Meyer K, Ray R (1996) Hepatitis C virus core protein cooperates with ras and transforms primary rat embryo fibroblasts to tumorigenic phenotype. J Virol 70: 4438-4443.
- Ray RB, Meyer K, Ray R (1996) Suppression of apoptotic cell death by hepatitis C virus core protein. Virology 226: 176-182.
- Ray RB, Steele R, Meyer K, Ray R (1997) Transcriptional repression of p53 promoter by hepatitis C virus core protein. J Biol Chem 272: 10983-10986.
- Ruggieri A, Harada T, Matsuura Y, Miyamura T (1997) Sensitization to Fas-mediated apoptosis by hepatitis C virus core protein. Virology 229: 68-76.
- Yoshida H, Kato N, Shiratori Y, Otsuka M, Maeda S, et al. (2001) Hepatitis C virus core protein activates nuclear factor kappa B-dependent signaling through tumor necrosis factor receptor-associated factor. J Biol Chem 276: 16399-16405.
- Taniguchi H, Kato N, Otsuka M, Goto T, Yoshida H, et al. (2004) Hepatitis C virus core protein upregulates transforming growth factor-beta 1 transcription. J Med Virol 72: 52-59.
- Banerjee A, Ray RB, Ray R (2010) Oncogenic potential of hepatitis C virus proteins. Viruses 2: 2108-2133.
- Kanda T, Steele R, Ray R, Ray RB (2008) Hepatitis C virus core protein augments androgen receptor-mediated signaling. J Virol 82: 11066-11072.
- Kanda T, Basu A, Steele R, Wakita T, Ryerse JS, et al. (2006) Generation of infectious hepatitis C virus in immortalized human hepatocytes. J Virol 80: 4633-4639.
- Yoshida T, Hanada T, Tokuhisa T, Kosai K, Sata M, et al. (2002) Activation of STAT3 by the hepatitis C virus core protein leads to cellular transformation. J Exp Med 196: 641-653.
- Bouchard MJ, Schneider RJ (2004) The enigmatic X gene of hepatitis B virus. J Virol 78: 12725-12734.
- Kanda T, Yokosuka O, Imazeki F, Yamada Y, Imamura T, et al. (2004) Hepatitis B virus X protein (HBx)-induced apoptosis in HuH-7 cells: influence of HBV genotype and basal core promoter mutations. Scand J Gastroenterol 39: 478-485.
- Kanda T, Yokosuka O, Nagao K, Saisho H (2006) State of hepatitis C viral replication enhances activation of NF-kB- and AP-1-signaling induced by hepatitis B virus X. Cancer Lett 234: 143-148.
- Zheng Y, Chen WL, Ma WL, Chang C, Ou JH (2007) Enhancement of gene transactivation activity of androgen receptor by hepatitis B virus X protein. Virology 363: 454-461.
- Chiu CM, Yeh SH, Chen PJ, Kuo TJ, Chang CJ, et al. (2007) Hepatitis B virus X protein enhances androgen receptor-responsive gene expression depending on androgen level. Proc Natl Acad Sci U S A 104: 2571-2578.
- Yang WJ, Chang CJ, Yeh SH, Lin WH, Wang SH, et al. (2009) Hepatitis B virus X protein enhances the transcriptional activity of the androgen receptor through c-Src and glycogen synthase kinase-3beta kinase pathways. Hepatology 49: 1515-1524.
- Wu MH, Ma WL, Hsu CL, Chen YL, Ou JH, et al. (2010) Androgen receptor promotes hepatitis B virus-induced hepatocarcinogenesis through modulation of hepatitis B virus RNA transcription. Sci Transl Med 2: 32ra35.
- Wang SH, Yeh SH, Lin WH, Wang HY, Chen DS, et al. (2009) Identification of androgen response elements in the enhancer I of hepatitis B virus: a mechanism for sex disparity in chronic hepatitis B. Hepatology 50: 1392-1402.
- Ma WL, Hsu CL, Wu MH, Wu CT, Wu CC, et al. (2008) Androgen receptor is a new potential therapeutic target for the treatment of hepatocellular carcinoma. Gastroenterology 135: 947-955, 955.
- Li Z, Tuteja G, Schug J, Kaestner KH (2012) Foxa1 and Foxa2 are essential for sexual dimorphism in liver cancer. Cell 148: 72-83.
- Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, et al. (2007) Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 317: 121-124.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 15064
- [From(publication date):
specialissue-2013 - Nov 30, 2025] - Breakdown by view type
- HTML page views : 10369
- PDF downloads : 4695