An Analysis of the Expression of Bcl-2, Podoplanin and Lymph Angiogenesis in Benign and Malignant Salivary Gland Tumours
Received: 12-Jun-2013 / Accepted Date: 04-Sep-2013 / Published Date: 06-Sep-2013 DOI: 10.4172/2161-0681.1000145
Abstract
Background: Salivary gland tumours comprise a significant proportion of oral tumours and are the next common neoplasm of the mouth after squamous cell carcinoma. These neoplasm’s have widely variable histopathologic and biologic characteristics, which makes it difficult to determine the pathogenesis and selection of therapeutic modalities. Alteration in some proto-oncogenes and tumour suppressor genes may lead to the development and progression of these tumours.
Aim: The purpose of this study was to analyze the immune histochemical expression of Bcl-2 and epithelial podoplanin and lymph angiogenesis in benign and malignant salivary gland tumours.
Material and method: The sample consisted of 20 formalin-fixed paraffin embedded blocks of benign and malignant salivary glands tumours. Immunohistochemical staining procedure was performed using monoclonal anti Bcl-2 antibody and D2-40 antibody. The sections were evaluated for Bcl-2 and epithelial podoplanin expression and D2-40 positive lymphatic vessels.
Results and conclusion: Bcl-2 showed positive immune expression in all benign and malignant salivary gland tumours with mucoepidermoid carcinomas showing most intense expression. Podoplanin immunostaining was also assessed, highest recorded score was in peripheral mucoepidermoid carcinoma (10.4? 3.05). Lymphatic micro vessel density expressed by podoplanin showed intense score in central mucoepidermoid carcinoma (5.05? 5.0). Present investigation demonstrated positive Bcl-2, podoplanin response and Lymphatic micro vessel density in both benign and malignant salivary gland tumours suggesting that Bcl-2 and podoplanin alteration may be associated with the progression of these neoplasms.
Keywords: Bcl-2; Podoplanin; Apoptosis; Lymph angiogenesis; Lymphatic micro vessel density
311099Introduction
Salivary gland tumours comprise a significant proportion of oral tumours and are the next common neoplasm of the mouth after squamous cell carcinoma. These neoplasm’s have widely variable histopathologic and biologic characteristics, which makes it difficult to determine the pathogenesis and selection of therapeutic modalities [1].
Apoptosis (programmed cell death) is a specific form of cell death that constitutes an important mechanism of maintaining homeostasis. Apoptosis occurs physiologically as well as in the course of many diseases. Alterations of apoptosis are always coupled with pathological conditions and/or oncogenesis [2]. Anti-apoptotic marker like Bcl-2 may potentially be able to predict the tumour behaviour.
Bcl-2 encoded by the proto-oncogene Bcl-2 and expressed in many types of malignant tumours, protect cells from apoptosis induced DNA damaging agents. This anti-apoptotic effect is assumed to be caused by retardation of cell proliferation due to cell’s accumulation in the G0 and G1 phases of the cell cycle [3].
Bcl-2 contributes to malignant cell expansion primarily by prolonging cell survival rather than by increasing the rate of cellular proliferation, and accumulation of cells with an aberrant Bcl-2 expression could be an important step in carcinogenesis [4,5]. Its over expression has been reported in most human low grade tumours and this inhibition of apoptosis has been regarded as being one of the most common pathways of tumourigenesis [6].
Invasion of cells into the surrounding tissue and destruction of normal tissue architecture are two hallmarks of malignant tumours. Morphologically, two patterns of tumour invasion can be distinguished: single cell and collective cell invasion. Podoplanin, a small mucin like protein, mediates a pathway leading to a collective cell migration in vivo and in vitro. Human podoplanin is a 38KDa type-I trans membrane glycoprotein consisting of 162 amino acids, nine of which form the intercellular domain. In normal human tissue, podoplanin is expressed in kidney podocytes, in skeletal muscle, placenta, lung and heart, in my fibroblasts of the breast and salivary glands, in osteoblasts and mesothelial cells [7]. D2-40 a commercially available antibody specifically recognizes human podoplanin and can be used to assess podoplanin expression in tumour cells [8]. Podoplanin expression using D2-40 has been reported to appear in many types of human cancers such as mesothelioma [9], skin cancer, carcinoma of the uterine cervix [10], ovarian cancer [11], thyroid cancer and head and neck squamous cell carcinoma [12].
Podoplanin is highly and superficially expressed in lymphatic endothelial cells, but not blood vessel endothelium. The immunostaining of D2-40 is now widely used for the detection of tumour lymph angiogenesis in many human cancers.
Thus, this study was attempted to investigate the immunohistochemical expression of Bcl-2 and podoplanin in the tumour cells of benign and malignant salivary gland tumours and to assess lymph angiogenesis in these lesions.
Materials and Methods
A total of 20 formalin fixed, paraffin embedded blocks of previously diagnosed cases of benign and malignant salivary gland tumours were selected from the archives of Department of Oral and Maxillofacial Pathology, M.M College of Dental Sciences and Research, Mullana, Ambala, to analyze immunohistochemical expression of Bcl-2 and podoplanin (D2-40). The sample comprised of 8 benign tumours (pleomorphic adenoma=8) and 12 malignant tumours (mucopeidermoid carcinoma=9, adenocarcinoma NOS=2, Polymorphous low grade adenocarcinoma=1).
Serial sections of 4µm thickness were taken on silanised slides meant for immunohistochemistry. All slides were then subjected to microwave heat- induced epitope retrieval (cycle 1: 98°C for 5 minutes, cycle 2: 95°C for 8 minutes). Endogenous peroxidise activity was blocked by treating the sections for 10-15 minutes in 0.3% peroxide block solution. Power block was applied for 10-15 minutes and slides were then incubated with primary antibody, anti Bcl-2 (for Bcl-2) antibody and D2-40 antibody (for podoplanin) for 1 hour in a humidifying chamber and incubated with secondary monoclonal antibody for 30 minutes. DAB was applied to all slides till brown colour appeared and counterstained with haematoxylin.
Evaluation of Bcl-2 immunohistochemical staining
All slides were assessed at a magnification of 40X of the light microscope (Eclipse 80i) in 5 representative areas called hot spots of the epithelium. Yellow to dark brown particles in the cytoplasm, indicated positive staining evaluation of positive reactions was performed using the criteria defined by Eslami B. et al. [12]. According to him, cumulative points were calculated as a product of Staining Intensity Scores (A) and Proportion of Positively Stained Cell Score (B).
The staining intensity (A) was rated on a scale of 0 to 3 as score 0 (colourless), score 1 (light yellow), score 2 (brown yellow), score 3 (brown). An average of 5 hotspots was then as the staining intensity (A) of the slide. Proportion of stained tumour cells (B) was scored as score 1 (when less than 25% positive cells), score 2 (25 - 75% positive cells) and score 3 (when more than 75% positive cells). The scores were based on examination of the whole section in each biopsy. Cumulative score was then calculated (A x B) and scored as score: 0 negative (-), score: 1-4 weakly positive (+) and score : >4 strongly positive (++).
Evaluation of D2-40 positive tumour cells
Cytoplasm and/or membrane immune reactivity were considered to indicate D2-40 expression. Analysis of positivity of epithelium was performed under 20X objective and 10X ocular lens (X200 magnification). The scores were based on examination of the whole section in each biopsy and immunostaining was scored under the following criteria. Quantitative scores of 0 to 5 were given as Score 0 (0% of tumour cells are positive), Score 1 (1% to 10% of tumour cells are positive), Score 2 (11% to 30% of tumour cells are positive), Score 3 (31% to 50% of tumour cells are positive), Score 4 (51% to 80% of tumour cells are positive) and Score 5 (81% to 100% of tumour cells are positive). The staining intensity is rated on a scale of 0 to 3 as score 0 (negative), score 1 (weak), score 2 (moderate) and score 3 (strong). The data was then converted to a German Immunoreactive Score (IRS) as a product of Quality Score and Staining Intensity Scores. IRS score was calculated as score: 8 or higher (Highly reactive), score: 4 to 7 (Moderately reactive) and score: 0 to 3 (Weakly reactive).
Quantification of lymphatic micro vessel density (LMVD)
For evaluating the lymphatic micro vessel density (LMVD) immunohistochemical D2-40 reactions were evaluated considering the cytoplasmic staining in lymphatic endothelial cells. Evaluation of positive reactions was performed by counting positive D2-40 lymphatic vessels, sitting around a visible lumen clearly separated from adjacent micro vessels and from other connective tissue components. Packed vessels were assumed as one lymphatic unit. The images were captured on to computer under 20X objectives, to evaluate the number of lymphatics. All the results were then statistically analyzed using ‘T test’, ‘Mann Whitney test’ and ‘Chi - square test’.
Results
Bcl-2 showed positive immune expression in both benign and malignant salivary gland tumours. In benign neoplasm’s, all cases showed weak immune expression. (Mean=1.8 ± 0.64) (Table 1). In malignant neoplasms, expression varied from weak to intense with 1 of 5 cases of peripheral MEC showing strong positive expression (Mean=3.4 ± 1.95). All 4 cases of central type showed weak expression (Mean=2 ± 1.414). Other cases of malignant neoplasm’s studied (adenocarcinoma NOS, polymorphous low grade adenocarcinoma) also showed weak Bcl-2 immune expression. Overall, malignant tumours showed a more intense Bcl-2 immune expression as compared to benign tumours. (Mean=2.58 ± 1.72) (Table 1).
| S.No | Lesion | No. of cases | No. of positive cases | Cumulative score | |||
|---|---|---|---|---|---|---|---|
| Negative | Weakly positive | Strongly positive | Mean ± SD | ||||
| 4-Jan | >4 | ||||||
| 0 | |||||||
| Group I | |||||||
| Benign | Pleomorphic adenoma | 8 | 8 | - | 8 | - | 1.8 ± 0.64 |
| Malignant | Peripheral mucoepidermoid carcinoma | 5 | 5 | - | 4 | 1 | 3.4± 1.95 |
| Central mucoepidermoid carcinoma | 4 | 4 | - | 4 | 2 ± 1.414 | ||
| Adenocarcinoma nos | 2 | 2 | - | 2 | - | 2.5 ± 2.121 | |
| PLGA | 1 | 1 | - | 1 | - | 1 | |
| Total | 12 | 12 | - | 11 | 1 | 2.58 ± 1.72 | |
Table 1: Malignant tumours showing more intense Bcl-2 immune expression as compared to benign tumours.
All cases of salivary gland neoplasm have showed positive podoplanin immune expression. In benign neoplasm’s, expression varied from weak to intense with 3 out of 8 cases showing strong podoplanin immune expression (total mean=5.88 ± 3.271) (Table 2). Malignant neoplasms also expression of podoplanin varied from weak to high. 1 case of polymorphous low grade adenocarcinoma studied showed weak expression. (Mean=2) All 5 cases of peripheral mucoepidermoid carcinoma studied showed intense expression (mean=10.44 ± 3.05). 1 out of 4 cases of central mucoepidermoid carcinoma showed high immune expression and 3 cases showing moderate expression. (Total mean=6.50 ± 3.786). 1 of 2 cases of adenocarcinoma NOS showed intense expression (mean=6 ± 2.828). Malignant neoplasm’s showed higher mean of podoplanin expression (7.66 ± 3.91) as compared to benign neoplasm (5.88 ± 3.271) (Table 2).
| S no. | Lesion | No. Of total cases | No. Of positive cases | Cumulative score | |||
|---|---|---|---|---|---|---|---|
| Weakly reactive | Moderately reactive | Highly reactive | Mean ± sd | ||||
| 0-3 | 7-apr | >8 | |||||
| Group I | Plemorphic adenoma | ||||||
| Benign | 8 | 8 | 3 | 2 | 3 | 5.88 ± 3.271 | |
| Malignant | Peripheral mucoepidermoid carcinoma | 5 | 5 | - | - | 5 | 10.4 ± 3.05 |
| Central mucoepidermoid carcinoma | 4 | 4 | - | 3 | 1 | 6.50 ± 3.786 | |
| Adenocarcinoma nos | 2 | 2 | - | 1 | 1 | 6 ± 2.828 | |
| PLGA | 1 | 1 | 1 | - | - | 2 | |
| Total | 12 | 12 | 1 | 4 | 7 | 7.66 ± 3.91 | |
Table 2: Cases showing strong podoplanin immune expression.
Lymphatic vessel density (LMVD) expressed by podoplanin in benign neoplasm was significant (5.1 ± 3.13). Lymph angiogenesis was also pronounced in MEC and present in both types. Peripheral MEC showed a mean LMVD count of (4.2 ± 2.37) whereas central MEC showed higher count of mean (5.05 ± 5.021) (Table 3). The number of podoplanin positive lymphatic vessels was not higher in adenocarcinoma NOS than other salivary gland tumours (Mean=2.60 ± 1.697). Both, Bcl-2 and podoplanin were weakly expressed in polymorphous low grade adenocarcinoma but it showed a significant LMVD count (Mean=3.40) (Table 3). Results were compared to haematoxylin, eosin stained sections of same tissues, and only those vessels, which were recognized as endothelial cell, lined vessels in H & E stained sections were counted as positive lymphatic vessels.
| Groups | Lmvd |
|---|---|
| Benign tumours | |
| Pleomorphic adenoma | 5.1 ± 3.13 |
| Malignant tumours | |
| Peripheral mucoepidermoid carcinoma | 4.2 ± 2.37 |
| Central mucoepidermoid carcinoma | 5.05 ± 5.021 |
| Adenocarcinoma nos | 2.60 ± 1.697 |
| PLGA | 3.4 |
| Total | 4.15 ± 3.15 |
Table 3: Peripheral MEC showed a mean LMVD count whereas central MEC showed higher count of mean.
Discussion
Tumour initiation, progression and invasion involve cellular proliferation, apoptosis as well as cell adhesion and communication that ensure cell survival, renewal and co-ordination. Salivary gland neoplasms show a varied behaviour [7]. Benign salivary gland neoplasm’s like pleomorphic adenomas may show an indolent behaviour or grow to very large sizes with/without any signs of malignant changes. Malignant salivary gland tumours may also show a varied behaviour ranging from borderline malignancies like PLGA to high grade MECs. Thus, in the present study, these neoplasms were assessed for the expression of Bcl-2, an anti-apoptotic marker; and podoplanin, a small mucin like protein, which has been shown to mediate cell migration and invasion in various malignancies. An increase in number of lymphatic vessels in the tumours trauma has also been shown to correlate with lymph node metastasis and is a predictor of clinically meaningful outcomes in a number of malignancies. Thus, the role of lymph angiogenesis in salivary gland neoplasm was also assessed.
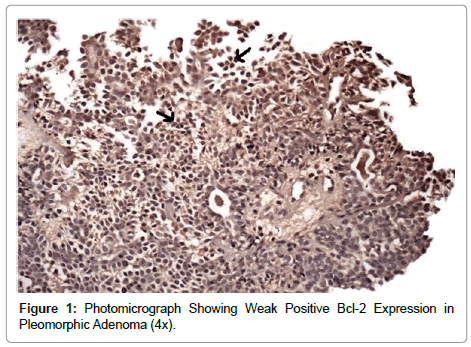
In the present study, all the 20 cases (100%) of salivary gland neoplasms showed positivity for Bcl-2 immune expression. Benign salivary gland tumour which included 8 cases of pleomorphic adenomas showed weakly positive Bcl-2 expression. This immune expression was seen mainly in ductal cells (Figure 1). Studies have also shown positive staining for Bcl-2 in pleomorphic adenomas, indicating the possible role of Bcl-2 as an anti-apoptotic agent in these neoplasm’s [13]. In a study performed by Yanez et al. [14] the immunohistochemical gene Bcl-2 protein expression was determined in 27 salivary gland tumours. They found that Bcl-2 protein could be expressed in virtually all benign and malignant salivary gland tumours. This suggests Bcl-2 protein has important role in the development of this tumour [14].
In the present study, the immunohistochemical localization of Bcl-2 in malignant tumours varied from weak to intense with mucopeidermoid carcinomas showing most intense expression followed by adenocarcinoma NOS and polymorphous low grade adenocarcinoma. Soini et al. [15] compared Bcl-2 expression in pleomorphic adenomas and in malignant salivary gland tumours comprising mainly of Adenoid cystic carcinoma and Mucoepidermoid carcinoma and found 100% positivity in pleomorphic adenoma and 64% positivity in malignant tumours [15]. They attributed 100% positivity in pleomorphic adenoma to the large size and long duration of the tumour at the time of diagnosis, thus emphasizing the association of increased cell survival with increased Bcl-2 expression. In addition, cell survival is generally more in malignant neoplasms than benign neoplasms. The benign neoplasm (pleomorphic adenomas) in this study showed overall weaker expression of Bcl-2 when compared to malignant salivary gland tumours. PLGA showed generalized weak expression, which may be associated with the low grade malignant potential of the neoplasm.
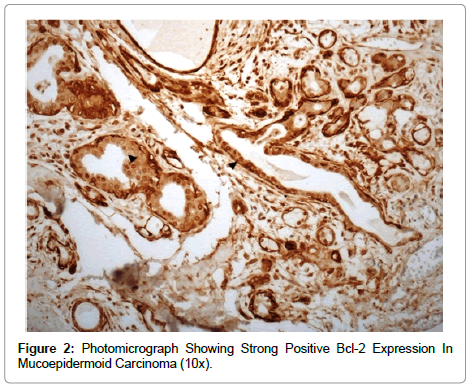
A recent report of Bcl-2 expression in MEC by Yin et al concluded that Bcl-2 is one of the potentially useful markers for survival in patients with MEC in minor salivary glands. The expression of Bcl-2 in cells in MEC may be linked to the degree of differentiation. Terminal differentiated cells like the mucous cells, normal acinar cells, salivary gland duct cells also do not express Bcl-2, but basal cells of the normal oral epithelium express Bcl-2 [16]. In our study, Bcl-2 immunohistochemical localization in malignant tumours mostly showed weak expression with only 1 out of 7 mucoepidermoid carcinomas showing strongly positive expression. Immunoreactivity was mainly found in the peripheral tumour cells and cells surrounding tubulo-ductal structures (Figure 2).
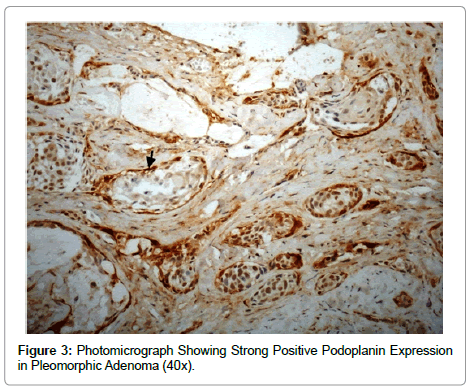
In the present study, all cases of salivary gland neoplasm’s showed positive podoplanin expression in the tumour cells. All pleomorphic adenomas studied in this case showed positivity for podoplanin expression. 3 out of 8 cases of pleomorphic adenoma showed high staining intensity and 2 cases showed moderate staining intensity. Podoplanin immunoreactivity was mainly seen in myopeithelial cells, also seen in cells surrounding the tubulo-ductal structures (Figure 3). Kanner [17] in their study showed that myopeithelial cells in breast and salivary gland and basal cells in prostrate consistently demonstrate podoplanin immune reactivity, but typically less intensely than lymphatic’s. Cutaneous and mucosal based basal cells also showed podoplanin expression but often in a patchy, focal manner [17].
Salivary gland myoepithelial cells have been reported to constantly produce podoplanin and glycosylate with polysaccharides in the Golgi apparatus, and transport it to the cell membrane. Podoplanin may be involved in maintaining the homeostasis of myoepithelial cells through its characteristic mucin type transmembrane protein. Various findings have suggested that podoplanin is an antigen for salivary gland myoepithelial cells and that the immunostaining of podoplanin in salivary glands directly reflects myoepithelial cell shapes [18].
The immunohistochemical expression of podoplanin in malignant tumours varied from weak to intense with mucopeidermoid carcinomas showing the most intense expression followed by adenocarcinoma NOS and polymorphous low grade adenocarcinoma.
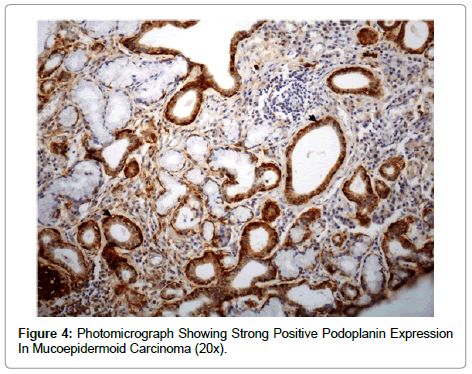
It was found that 5 peripheral mucopeidermoid carcinomas showed high expression for podoplanin and 3 out of the 4 central mucoepidermoid carcinomas showed moderate expression suggesting a more intense expression of podoplanin in peripheral than central MECs. Positive podoplanin expression in MECs was restricted to the epidermoid cells while the mucous cells did not show any podoplanin expression. The intermediate cells showed a variable staining pattern (Figure 4). Podoplanin has been reported to enhance tumour invasion by enhancing cell motility. Cancer cell migration and invasion involves active remodelling of actin cytoskeleton, however, podoplanin does not interact directly with actin but via ERM proteins such as ezrin, radixin and moesin. It has been shown that over expression of podoplanin leads to increased phosphorylation of ezrin, which links to the observed rearrangement of actin cytoskeleton. Podoplanin also increases activity of Rho family GTPases, which have also been linked to tumour cell motility [19]. It was also found both the cases of Adenocarcinoma NOS showed positive podoplanin expression with 1 showing pronounced expression.
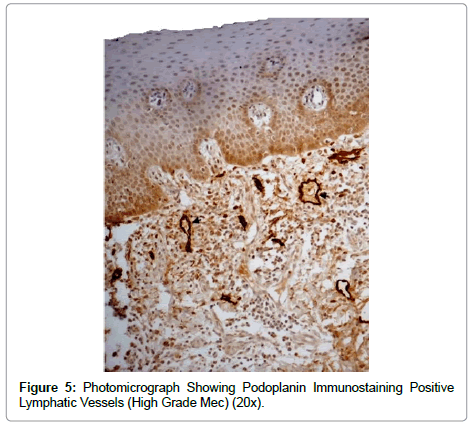
In addition, lymphatic vessel density (LMVD) expressed by podoplanin in pleomorphic adenomas was significant suggesting lymphangiogensis to be pronounced in this neoplasm. Lymph angiogenesis was also pronounced in MEC and present in both types (Figure 5). Mean of lymphatic micro vessel density count was found to be almost similar in both central and peripheral MEC. An increase in number of lymphatic vessels in the tumour trauma has also been shown to correlate with lymph node metastasis and is a predictor of clinically meaningful outcomes in a number of malignancies. Adenocarcinoma NOS showed LMVD counts similar to MECs. Though, Bcl-2 and D2- 40 were weakly expressed in polymorphous low grade adenocarcinoma but it showed a significant LMVD count.
Thus, the present investigation demonstrated positive Bcl-2, podoplanin response and LMVD in both benign and malignant salivary gland tumours suggesting that Bcl-2 and podoplanin alteration may be associated with the progression of these neoplasms. But no statistically significant difference was observed with regard to intensity of Bcl- 2 and podoplanin staining in benign and malignant salivary gland tumours. Studies of a larger sample with a wide variety of benign as well as malignant salivary gland tumours comprising of all subtypes or variants of tumours are required to understand the significance of Bcl- 2 and podoplanin expression in the prognosis and clinical outcome for the future generations.
References
- Natheer H, Omer H, Kawas S (2010) Immunohistochemical analysis of p53 and bcl-2 in benign and malignant salivary gland tumours. J Oral Pathol Med 39: 48-55.
- Borowska B, Filip A, Wojcierowski J, Smolen A, Korobowicz E, et al. (2006) Estimation of prognostic value of bcl-a L gene expression in non-small lung cancer. Lung Cancer 51:61-69.
- Dijikema IM, Struimans H, Dullens HFJ, Kal HB, Van der Well I, et al. (2000) Influence of pb53 and bcl-2 on proliferative activity and treatment outcome in head and neck cancer patients. Oral Oncol 36: 54-60.
- Lo Muzio L, Staibano S, Pannone G, Bucci P, Nocini PF, et al. (1999) Expression of cell cycle and apoptosis related proteins in sporadic odontogenic keratocysts and odontogenic keratocysts associated with the naevoid basal cell carcinoma syndrome. J Dent Res 78: 1345-1353.
- Hamsel BT, Smedts F, Kuijpers J, Jeunink M, Trimbos B, et al. (1996) Bcl-2 immunoreactivity increases with severity of CIN: a study of normal cervical epithelia, CIN and cervical carcinoma. J Pathol 179:26-30.
- Ramsay JA, From L, Kahn HJ (1995) Bcl-2 protein expression in melanocytic neoplasms of the skin. Mod Pathol 8: 150-154.
- A Wicki, G Christofori (2007) The potential role of podoplanin in tumor invasion. Brit J Cancer 96: 1-5.
- Kadota GK, Huang CL, Liua D, Nakashimaa N, Yokomisea H, et al. (2010) The clinical significance of the tumor cell D2-40 immunoreactivity in non small cell lung cancer. Lung Cancer 70: 88-93.
- Mehkri S, Iyengar AR, Nagesh KS, Bharati MB (2010) Analysis of cell proliferation rate in Oral Leukoplakia and Oral Squamous Cell Carcinoma. J Clin Exp Dent 2: 173-177.
- Eslami B, Yaghmaei M, Firoozi M, Saffar AS (2003) Nucleolar organizer regions in selected odontogenic lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 95: 187-192.
- Kenji K, Kumamoto H, Ooya K (2000) Analysis of Apoptosis-related Factors and Apoptotic Cells in Lining Epithelium of Odontogenic Keratocysts. Oral Medicine & Pathology 5: 35-40.
- Eslami B, Rahimi H, Rahimi F, Khiavi MM, Ebadifar A (2006) Diagnostic value of silver nitrate staining for nucleolar organizer regions in selected head and neck tumours. J Can Res Ther 2: 129-131.
- Aoki T, Tsukinoki K, Karakida K, Ota Y, Otsura M, et al. (2004) Expression of cyclo-oxygenase 2, bcl-2 and Ki-67 in pleomorphic adenoma with special reference to tumor differentiation and apoptosis. Oral Oncol 40: 954-959.
- Yanez M, Roa I, Garcia M, Ibacache G, Villaseca M (1999) Bcl-2 gene expression in salivary gland tumours. Rev Med Chil 127: 139-142.
- Soini Y, Törmänen U, Pääkkö P (1998) Apoptosis is inversely related to bcl-2 but not to bax expression in salivary gland tumours. HistopaÂthology 32: 28-34.
- Yin HF, Okada N, Takagi M (2000) Apoptosis and apoptotic related factors in mucoepidermoid carcinoma of the oral minor salivary glands. Pathol Int 50: 603-609.
- Kanner W A, Galgano M T, Atkins K A (2010) Podoplanin expression in basal and myoepithelial cells: utility and potential pitalls. Appl Immunohistochem Mol Morphol 18: 226-230.
- Hata M, Amano I, Tsuruga E, Kojima H, Sawa Y (2010) Immunoelectron Microscopic Study of Podoplanin Localization in Mouse Salivary Gland Myoepithelium. Acta Histochem Cytochem 43: 77-82.
- Raica M, Cimpean AM and Ribatti D (2008) The role of podopalnin in tumor progression and metastasis. Anticancer Res 28: 2997-3006.
Citation: Kaur H, Gupta S (2013) An Analysis of the Expression of Bcl-2, Podoplanin and Lymph Angiogenesis in Benign and Malignant Salivary Gland Tumours. J Clin Exp Pathol 3:145. DOI: 10.4172/2161-0681.1000145
Copyright: © 2013 Kaur H, et al.. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 15241
- [From(publication date): 11-2013 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 10564
- PDF downloads: 4677