Review Article Open Access
Alzheimer's Disease-Related Amyloidopathy in Visual Impairment
Xiao Li1,2 and Can Zhang1*1Genetics and Aging Research Unit, Mass General Institute for Neurodegenerative Diseases (MIND), Department of Neurology, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA, 02129-2060, USA
2Department of Ophthalmology, General Hospital of the People's Liberation Army, 28 Fuxing Road, Beijing, 100853, P.R. China
- *Corresponding Author:
- Can (Martin) Zhang, M.D/Ph.D
Harvard Medical School
Genetics and Aging Research Unit
Mass General Institute for Neurodegenerative Disease
Massachusetts General Hospital
114 16th Street Charlestown, MA 02129, USA
Tel: 617-726-6845;
Fax: 617-724-1949;
E-mail: zhang.can@mgh.harvard.edu
Received July 14, 2012; Accepted August 28, 2012; Published August 31, 2012
Citation:Li X, Zhang C (2012) Amyloidopathy in the Eye of Alzheimer's Disease. J Addict Res Ther S5:005. doi:10.4172/2155-6105.S5-005
Copyright: © 2012 Li X, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Addiction Research & Therapy
Abstract
Alzheimer’s disease (AD) is a devastating neurodegenerative disease and the primary cause of dementia, with no cure available. The pathogenesis of AD is believed to be primarily driven by the accumulation of Aβ, a 4-kDa peptide generated from the amyloid-β precursor protein (APP) through proteolysis. APP is a type-I trans-membrane protein which is constitutively expressed in many tissues, including the eye. Emerging evidence support that visual impairment and several common eye disorders may share common pathogenic determinants with AD. Over the past decade, an increasing number of researches have been utilizing mouse models to investigate the underlying mechanisms of human disorders. Intriguingly, AD animal models present devastating amyloidopathy not only in the brain but also in the eye. This article aims to describe the progress in understanding the pathophysiology and pathogenesis of AD, focusing on the amyloidopathy in visual impairment. Moreover, the studies described in this review support the potential use of non-invasive ocular tests for screening AD patients at an earlier stage and for assessing treatment efficacy of AD.
Keywords
Alzheimer’s disease; Amyloid-β; Amyloidopathy; Amyloid-β precursor protein; Tau; Visual deficits; Retina; Lens; Animal model
Introduction
AD is a devastating neurodegenerative disease and is the primary cause of dementia. Amyloidopathy and tauopathy underlie the two pathological hallmarks in AD brain [1-4]. Recently, many reports describe frequent involvement of visual disturbance and the role of amyloidopathy in the eye impairment of AD patients [5-7]. Interestingly, considerable studies using AD animal models demonstrate devastating amyloidopathy not only in the brain but also in the eye, supporting amyloidopathy in the pathology of visual impairment. This study aims to describe the progress in understanding the pathophysiology and pathogenesis of AD, focusing on the amyloidopathy in the eye. We will first describe the background of AD, the visual changes in AD patients, the involvement of amyloidopathy in several common eye disorders, and then discuss the involvement of amyloidopathy in the eye of AD mouse models.
Basics of Alzheimer's Disease (AD)
The primary clinical features of AD are characterized by deterioration of memory and cognitive function, progressive impairment of activities of daily living, and several neuropsychiatric symptoms [1]. On the cell and molecular levels, the pathophysiology of AD is characterized by two distinctive features: amyloid plaques comprised primarily of a small peptide named Aβ [2-4], and neurofibrillary tangles composed of hyperphosphorylated tau. While Aβ42 and Aβ40 are the two primary Aβ species, Aβ42 is more prevalent than Aβ40 in amyloid plaques. Considerable evidence from genetics, biochemistry and molecular biology supports the amyloid-cascade hypothesis stating that the production and excessive accumulation of Aβ is the primary pathological event leading to AD [2,8]. Specifically, accumulation and aggregation of Aβ can induce a series of toxicity-mediated "gain-offunctional" activities including inflammatory responses, followed by hyperphosphorylation of the tau protein, formation of fibrillary tangles, and activation of apoptotic pathways [2-4]. In parallel, these gained and often toxic activities contribute to "loss-of-functional" activities in proteasome and lysosome, as well as mitochondria [3,9-12]. Ultimately, these abnormal functional activities lead to neuronal dysfunction and cell death [3,9-12].
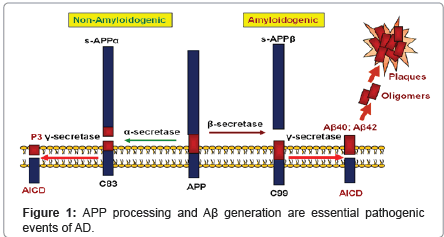
Aβ is produced via a serial cleavage of amyloid-β precursor protein (APP) by β-secretase (BACE1) and γ-secretase (Figure 1) [3,13-16]. APP is a type-I trans-membrane protein, that is constitutively expressed in many tissues. The initial cleavage of APP can occur through α- or β-secretase. α-Secretase cleavage produces sAPPα and the α-Cterminal fragment (α-CTF or C83); β-secretase cleavage produces sAPPβ and β-C-terminal fragment (β-CTF or C99). Following trophic factor deprivation, sAPPβ can be further cleaved by an unidentified protease, to produce N-APP, which contains the N-terminal 286 amino acids of APP [17,18]. C83 and C99 can be further cleaved by γ-secretase to produce P3 or Aβ.
One of the strongest support of "amyloid hypothesis" in AD is based on the genetic studies. Fundamentally, AD is a genetically complex disorder. Based on the age of onset, it has two primary forms: early or late-onset of AD. More than 200 fully penetrant mutations in the amyloid β-protein precursor (APP), presenilin 1 (or PSEN1), and presenilin 2 (PSEN2) have been identified for early-onset familial AD (FAD) (<60 years old; 5-10% cases) [3,8,19], whereas 90-95% cases are late-onset AD (>65 years old) and a variant (ε4) of the gene encoding apolipoprotein E (APOE) has been associated with this disease type [20]. To date approximately 80% of the late-onset AD genetic variance still remains elusive [21]. Recently several genome-wide association studies have identified several novel AD candidate genes [3,22]. Functional characterization of these AD candidate genes has confirmed the important pathogenic factors of AD, including amyloidopathy (by ATXN1 [23,24]) and immune responses (by CD33 [23,25]).
Visual Deficits in AD Patients
Considerable evidence shows that AD usually presents with visual deficits from the eye, in addition to the primary complaint of cognitive decline from the brain [1]. For example, one piece of evidence is that visual disturbance is often an early complaint of AD patients, which may occur earlier than memory impairment [5]. Particularly, the visual impairment include deficiency in color vision and blindness [6,7], visual field loss [26], and backward masking reduction [27]. The second piece of evidence is that there is a significance higher prevalence of visual deficits in AD patients compared to normal population [27]. Third, visual analyses show heterogeneity in visual deficiency in AD patients: they tend to have high vulnerability in pattern vision, moderate vulnerability in spatial vision, and low vulnerability in motion and flicker perception [27]. Next, numerous studies also reported various anatomical changes in AD eye, including the changes in the pupil (enhanced pupil response to cholinergic drops [26,28]), the lens (supra-nuclear cataract [29]), the retina (thinning in the retinal nerve fiber layer [7]) and the optic disc (pallor, cupping or thinning [6]).
Understanding the physiological functions of the visual system may shed light on the visual deficits commonly seen in AD patients. The eye is a delicate organ of the human body processing and communicating outside information into brain. During visual process, the outside visual information is communicated to the brain through a synchronized course. In particular, the input light from outside pass through the cornea, get focused by the lens and then reach the retina. The retina performs essential and complex functions conveying visual message into the brain. It contains ten distinct layers of neurons, e.g. the retinal ganglion cells (RGCs) layer generating action potentials, the photoreceptor layer containing rod and cone cells sensitizing the intensity of input light, and the retinal pigment epithelium [30]. More than 20 physiologically distinct types of RGCs have been reported. Using interrelated neuronal synapses, they operate synchronously by a set of parallel pathway and convey dynamic action potentials (carrying input visual information) through the optic nerve to the brain [5,30,31].
The underlying mechanism of visual deficits in AD has not been fully identified. The mechanism may be related to the same embryonic origin (neuro-ectoderm) for both the eye and brain [32,33]; thus they may suffer from common pathogenic triggers from genetic and environmental circumstances. The mechanism may also be associated with inflammatory reactions in both the eye and the brain. Increasing evidence shows that AD is characterized by a systemic inflammation [34-36], accompanying with the course of cognitive decline. These inflammatory reactions include increases in both acute and chronic systemic inflammatory activities, and increases in the levels of serum tumor necrosis factor alpha [34-36]. Additionally recent unbiased genome-wide association studies identified variance in genes, e.g. CD33 [23,25], which encode proteins involved in immune system, suggesting that immune response plays pivotal roles in AD. Thus the systemic immune responses may account for the functional deficits in both the eye and the brain who share the same embryonic origin.
Amyloidopathy Related to Human Eye Disorders
Besides the general complaints of visual deficits, AD patients have a significantly increased chance to affect with specific eye disorders, e.g. open-angle glaucoma (OAG) [37,38]. Emerging evidence shows the involvement of amyloidopathy in several eye disorders related to AD. For example, Aβ levels/plaques are significantly higher in the lens [29] and the retina [39] in AD group compared to control group. Amyloidopathy has also been identified in common visual disorders, e.g. age-related macular degeneration (AMD) and cataract. The several following paragraphs will review the involvement of amyloidopathy in these eye disorders.
Open-angle glaucoma (OAG)
Glaucoma is a chronic and degenerative neuropathy, often characterized by the loss of RGCs and the presentation of optic nerve cupping in pathology. Primary open-angle glaucoma (OAG) is classified by the appearance of the iridocorneal angle. It affects a large number of population, accounting for the second leading cause of blindness in the U.S. alone and the leading cause of blindness among African Americans in the world [40]. Evidence from independent research groups suggests that AD patients have an increased occurrence rate of glaucoma, particularly OAG [37]. Bayer et al. showed that the prevalence of probable glaucoma in AD patients (24.5%) was significantly higher than that of the control group (6.5%) [37]. Interestingly, all patients with glaucoma in the study were primary OAG. Subsequently, Tamura and colleagues obtained similar results in Japanese population [38]. They investigated whether the prevalence of OAG in AD patients differed from an age-matched control group in a Japanese population. They found that the prevalence of OAG (23.8%) in AD patients was significantly higher than that of the control population (9.9%) [38]. Collectively, these results suggest that common pathogenic factors may contribute to both AD and OAG [38].
Age-related macular degeneration (AMD)
Age-related Macular Degeneration (AMD) is the leading cause of central blindness in the elderly who are over 50 years old in the world [41]. AMD is pathologically characterized by the presence of drusen, which are extracellular deposits that accumulate beneath the retinal pigmented epithelium. Recent studies show that drusen also contains substantial Aβ species which display different conformations based on antibody reactivity, in addition to apolipoprotein E and various complement components, e.g. C5, C5b9 complex, and C3 fragments [42]. Antibodies, including 6E10 which recognizes the first 16 amino acids of AD Aβ (anti-Aβ[1-16]; DAEFRHDSGYEVHHQK) [43], showed strong immuno-activity in both AD brain senile plaque (anticipated; positive control) and drusen in the eye. The authors also utilized electron microscopy as an independent approach and identified amyloid fibrils in drusen [42]. Thus, drusen contains the same Aβ species as those in AD brain, suggesting that Aβ accumulation is a key pathogenic determinant linking the pathology in both AMD and AD [42].
Cataract
Besides AMD and OAG, cataract is another common eye disorder with aging as the primary risk factor. It is common in people over the age of 60 and is the leading cause of blindness worldwide [44]. Cataract is caused by a clouding in the lens often due to protein aggregation [44]. The clouding impairs the focusing of input light as well as transduction into the retina and then brain, resulting in vision loss and even blindness if not treated. Amyloidopathy in AD and normal lens/eye has been intensively investigated. Goldstein and colleagues utilized various approaches including mass spectrometry, immunohistochemistry, and immunogold electron microscopy to investigate the amyloid in the lens and eye comparing AD and control group [29]. The authors found that: 1) both Aβ40 and Aβ42 in the lens were constitutively expressed at concentrations comparable to brain; 2) Aβ accumulated in AD lenses, exclusively in the cytoplasm of supranuclear and deep cortical lens fiber cells. They also found that AD patients presented equatorial supranuclear cataract in the lens, which co-localized with Aβ immuno-reactivity and birefringent Congo Red staining. Finally, they found that synthetic Aβ bound α-crystallin B subunit, a “molecular chaperone” or a heat-shock protein highly expressed in the lens. The involvement of amyloidopathy in the cataract is further supported by a recent finding that Down syndrome patients also show supranuclear cataract in the lens, similar to AD [45]. Patients of Down syndrome, also known as trisomy 21, carry triplication of APP and usually present AD neuropathology in their 40s of age, e.g. cerebral Aβ accumulation/deposition and cognitive decline [45]. Thus, these results clearly show the significance of amyloidopathy in the pathology of cataract.
Amyloidopathy in the Eye of AD Mouse Models
Model organisms provide a powerful tool to identify and characterize the mechanistic causes and potential treatment/cure of human disorders, including AD. For example, different animal models have been developed to investigate AD, including zebrafish [46-48], Caenorhabditis elegans [49-51], Drosophila [52,53] and mouse [54-58]. Considerable evidence from these AD animal models shows amyloidopathy and cognitive decline, as well as visual system deficits, recapitulating the impairment in AD patients. Next, this article will describe several widely-utilized AD mouse models, focusing on the results in the eye, with an aim to review the essential role of amyloidopathy in visual deficits.
Tg2576 is one of the earliest and well-characterized AD transgenic mouse models [54-58]. It carries the APP Swedish mutation (Lys670Asn/Met671Leu) and features marked increases in Aβ levels and plaque burden in the brain, as well as age-related behavioral impairment, and synaptic deficits [54-58]. Several groups have shown the amyloidopathy in the eye of Tg2576 mice [59,60]. In one study, Dutescu and colleagues used 14 months old Tg2576 mice and found strong APP expression in the inner nuclear layer and ganglion cell layer of the retina, as well as in the lens and the cornea [59]. They also found weak APP expression in the photoreceptor and retinal pigmented epithelial cell layer. In addition, Western blot analysis using Tg2576 and control mouse samples showed the expression of full-length APP and several APP cleavage products are ranging 18-70 kDa in molecular weight from the retina and brain. The authors studied the retina and brain Aβ levels and found that both Aβ40 and Aβ42 levels from the retina were lower than those in the brain of Tg2576 mice. Finally, they found the levels of both Aβ40 and Aβ42 from the retina of Tg2576 mice were much higher than the control mice [59]. Subsequently, Liu and colleagues confirmed the strong expression of APP in the retina of Tg2576 mice [60]. They also performed Aβ active immunization via subcutaneous vaccinations on these mice and showed that vaccination reduced retinal Aβ deposits, however with a marked increase in retinal inflammation manifested by microglial infiltration and astrogliosis [60].
The APPswe/PS1dE9 mouse model is another well-characterized and widely-utilized AD transgenic mouse model, carrying two FAD mutations (the APP Swedish mutation [Lys670Asn/Met671Leu] and the PS1dE9 mutation) [61,62]. These mice present much earlier onset of memory deficits and more aggressive Aβ deposition in the brain; compared to Tg2576 mouse model [63]. Amyloidopathy has been reported in the retina of APPswe/PS1dE9 mice from several independent groups [39,64]. Ning et al. compared the temporal and spatial expression patterns of APP, Aβ deposits and inflammatory reactions in the retina of these mice [64]. They found age-dependent increases in APP expression and Aβ deposition in multiple layers of the retina. In addition, Aβ accumulation was accompanied by increased immuno-reactivity and apoptotic processes in the RGCs [64]. Very recently, another group validated the retina amyloidopathy in APPswe/PS1dE9 mice, and moreover, retinal plaques were detected earlier than in the brain [39]. They also performed an immune-therapy which significantly reduced brain and retinal Aβ plaque. Intriguingly, they achieved curcumin-based non-invasive optical imaging of retinal Aβ plaques in vivo with high resolution and specificity [39].
Concluding Remarks and Future Perspectives
Despite a relative lack of knowledge in the tauopathy and the APP proteolytic secretases in the eye, compared to the well-characterized amyloidopathy, there has been clearly an enormous progress in understanding the eye impairment associated with AD. AD is the primary cause of dementia with complex underlying mechanisms and it often presents concomitant visual impairment. Multidisciplinary studies from genetics, biochemistry, cell and molecular biology using cell-based models, animal-based models, as well as human specimen provide evidence that the pathogenesis of AD is primarily contributed by Aβ accumulation and deposition in the brain. Here we reviewed the visual deficits of AD and the involvement of amyloidopathy in several common visual disorders of human, and then we discussed the amyloidopathy in the eyes of several AD mouse models. Collectively, these studies discussed in this article suggest the significant role of Aβ in the pathogenesis of visual deficits and eye disorders and they also further support the amyloid hypothesis of AD.
Emerging evidence supports that the eye may provide a "window" in the screening, early diagnosis or monitoring the therapeutic efficacy of AD. Currently a definite diagnosis of AD can only be confirmed post-mortem and a pre-mortem diagnosis of AD is primarily dependent on the evaluations of cognitive and memory impairment [65,66]. Due to the shared amyloidopathy of the eye and brain in AD, developing non-invasive ocular analyses may advance the AD clinical practice. For example, based on the aggregation in the lens caused by the amyloid or other proteins [29,44], the quasi-elastic laser light scattering spectroscopy has been utilized to detect and monitor these aggregates in mouse models [67]. The other example is the study from Koronyo-Hamaoui and colleagues, who utilized curcumin to label Aβ plaque and developed a novel high-resolution retinal plaque imaging in live AD mice and post-mortem retinal samples of AD patients [39]. Thus, developing and utilizing non-invasive ocular tests, e.g. the quasi-elastic laser light scattering spectroscopy or probe-based retinal imaging may support the earlier screening of AD patients, monitoring the disease progress and assessing the therapeutic efficacy of AD.
Acknowledgements
This study was supported by a Visiting Scholar's Fund provided by the General Hospital of the People's Liberation Army of China (Dr. X. Li) and a NIH grant (1K99AG039482-01; Dr. C. Zhang). The sponsors played no role in the preparation and submission of the manuscript.
References
- Cummings JL (2004) Alzheimer's disease. N Engl J Med 351: 56-67.
- Hardy J,Selkoe DJ (2002) The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297: 353-356.
- Bertram L,Tanzi RE (2008) Thirty years of Alzheimer's disease genetics: the implications of systematic meta-analyses. Nat Rev Neurosci 9: 768-778.
- Gandy S (2005) The role of cerebral amyloid beta accumulation in common forms of Alzheimer disease. J Clin Invest 115: 1121-1129.
- Sadun AA, Borchert M, Devita E, Hinton DR, Bassi CJ (1987) Assessment of visual impairment in patients with Alzheimer's disease. Am J Ophthalmol 104: 113-120.
- Cronin-Golomb A, Sugiura R, Corkin S, Growdon JH (1993) Incomplete achromatopsia in Alzheimer's disease. Neurobiol Aging 14: 471-477.
- Pache M, Smeets CH, Gasio PF, Savaskan E, Flammer J, et al. (2003) Colour vision deficiencies in Alzheimer's disease. Age Ageing 32: 422-426.
- Tanzi RE, Bertram L (2005) Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell 120: 545-555.
- Selkoe DJ, American College of Physicians, American Physiological Society (2004) Alzheimer disease: mechanistic understanding predicts novel therapies. Ann Intern Med 140: 627-638.
- Wright AF (2005) Neurogenetics II: complex disorders. J Neurol Neurosurg Psychiatry 76: 623-631.
- Selkoe DJ (2001) Alzheimer's disease: genes, proteins, and therapy. Physiol Rev 81: 741-766.
- Tanzi RE, Bertram L (2001) New frontiers in Alzheimer's disease genetics. Neuron 32: 181-184.
- Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, et al. (1999) Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286: 735-741.
- Kimberly WT, Lavoie MJ, Ostaszewski BL, Ye W, Wolfe MS, et al. (2003) Gamma-secretase is a membrane protein complex comprised of presenilin, nicastrin, Aph-1, and Pen-2. Proc Natl Acad Sci U S A 100: 6382-6387.
- Sisodia SS, St George-Hyslop PH (2002) gamma-Secretase, Notch, Abeta and Alzheimer's disease: where do the presenilins fit in? Nat Rev Neurosci 3: 281-290.
- Zhang C, Saunders AJ (2007) Therapeutic targeting of the alpha-secretase pathway to treat Alzheimer's disease. Discov Med 7: 113-117.
- Nikolaev A, Mclaughlin T, O'leary DD, Tessier-Lavigne M (2009) APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature 457: 981-989.
- Zhang C, Browne A, Kim DY, Tanzi RE (2010) Familial Alzheimer's disease mutations in presenilin 1 do not alter levels of the secreted amyloid-beta protein precursor generated by beta-secretase cleavage. Curr Alzheimer Res 7: 21-26.
- Tanzi RE, Gusella JF, Watkins PC, Bruns GA, St George-Hyslop P, et al. (1987) Amyloid beta protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science 235: 880-884.
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, et al. (1993) Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A 90: 1977-1981.
- Gatz M, Reynolds CA, Fratiglioni L, Johansson B, Mortimer JA, et al. (2006) Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry 63: 168-174.
- Bertram L, Lill CM, Tanzi RE (2010) The genetics of Alzheimer disease: back to the future. Neuron 68: 270-281.
- Bertram L, Lange C, Mullin K, Parkinson M, Hsiao M, et al. (2008) Genome-wide Association Analysis Reveals Putative Alzheimer's Disease Susceptibility Loci in Addition to APOE. Am J Hum Genet 83: 623-632.
- Zhang C, Browne A, Child D, Divito JR, Stevenson JA, et al. (2010) Loss of function of ATXN1 increases amyloid beta-protein levels by potentiating beta-secretase processing of beta-amyloid precursor protein. J Biol Chem 285: 8515-8526.
- Naj AC, Jun G, Beecham GW, Wang LS, Vardarajan BN, et al. (2011) Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer's disease. Nat Genet 43: 436-441.
- Trick GL, Trick LR, Morris P, Wolf M (1995) Visual field loss in senile dementia of the Alzheimer's type. Neurology 45: 68-74.
- Sherod MG, Griffith HR, Copeland J, Belue K, Krzywanski S, et al. (2009) Neurocognitive predictors of financial capacity across the dementia spectrum: Normal aging, mild cognitive impairment, and Alzheimer's disease. J Int Neuropsychol Soc 15: 258-267.
- Miyashita N, Imai H, Mori H, Kodera M, Shirai T, et al. (1997) A 76-year-old man with loss of vision and dementia. No To Shinkei 49: 773-782.
- Goldstein LE, Muffat JA, Cherny RA, Moir RD, Ericsson MH, et al. (2003) Cytosolic beta-amyloid deposition and supranuclear cataracts in lenses from people with Alzheimer's disease. Lancet 361: 1258-1265.
- Field GD, Chichilnisky EJ (2007) Information processing in the primate retina: circuitry and coding. Annu Rev Neurosci 30: 1-30.
- Greschner M, Shlens J, Bakolitsa C, Field GD, Gauthier JL, et al. (2011) Correlated firing among major ganglion cell types in primate retina. J Physiol 589: 75-86.
- Scholes JH (1987) Developmental neurobiology: uncertainties in the retina. Nature 328: 114-115.
- Sernagor E, Eglen SJ, Wong RO (2001) Development of retinal ganglion cell structure and function. Prog Retin Eye Res 20: 139-174.
- Holmes C, Cunningham C, Zotova E, Woolford J, Dean C, et al. (2009) Systemic inflammation and disease progression in Alzheimer disease. Neurology 73: 768-774.
- Bonotis K, Krikki E, Holeva V, Aggouridaki C, Costa V, et al. (2008) Systemic immune aberrations in Alzheimer's disease patients. J Neuroimmunol 193: 183-187.
- Holmes C, El-Okl M, Williams AL, Cunningham C, Wilcockson D, et al. (2003) Systemic infection, interleukin 1beta, and cognitive decline in Alzheimer's disease. J Neurol Neurosurg Psychiatry 74: 788-789.
- Bayer AU, Keller ON, Ferrari F, Maag KP (2002) Association of glaucoma with neurodegenerative diseases with apoptotic cell death: Alzheimer's disease and Parkinson's disease. Am J Ophthalmol 133: 135-137.
- Tamura H, Kawakami H, Kanamoto T, Kato T, Yokoyama T, et al. (2006) High frequency of open-angle glaucoma in Japanese patients with Alzheimer's disease. J Neurol Sci 246: 79-83.
- Koronyo-Hamaoui M, Koronyo Y, Ljubimov AV, Miller CA, Ko MK, et al. (2011) Identification of amyloid plaques in retinas from Alzheimer's patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. Neuroimage 54: S204-217.
- Kwon YH, Fingert JH, Kuehn MH, Alward WL (2009) Primary open-angle glaucoma. N Engl J Med 360: 1113-1124.
- Fine SL, Berger JW, Maguire MG, Ho AC (2000) Age-related macular degeneration. N Engl J Med 342: 483-492.
- Isas JM, Luibl V, Johnson LV, Kayed R, Wetzel R, et al. (2010) Soluble and mature amyloid fibrils in drusen deposits. Invest Ophthalmol Vis Sci 51: 1304-1310.
- Wang R, Sweeney D, Gandy SE, Sisodia SS (1996) The profile of soluble amyloid beta protein in cultured cell media. Detection and quantification of amyloid beta protein and variants by immunoprecipitation-mass spectrometry. J Biol Chem 271: 31894-31902.
- Moreau KL, King JA (2012) Protein misfolding and aggregation in cataract disease and prospects for prevention. Trends Mol Med 18: 273-282.
- Moncaster JA, Pineda R, Moir RD, Lu S, Burton MA, et al. (2010) Alzheimer's disease amyloid-beta links lens and brain pathology in Down syndrome. PLoS One 5: e10659.
- Newman M, Verdile G, Martins RN, Lardelli M (2011) Zebrafish as a tool in Alzheimer's disease research. Biochim Biophys Acta 1812: 346-352.
- Xi Y, Noble S, Ekker M (2011) Modeling neurodegeneration in zebrafish. Curr Neurol Neurosci Rep 11: 274-282.
- Newman M, Wilson L, Camp E, Verdile G, Martins R, et al. (2010) A zebrafish melanophore model of amyloid beta toxicity. Zebrafish 7: 155-159.
- Ash PE, Zhang YJ, Roberts CM, Saldi T, Hutter H, et al. (2010) Neurotoxic effects of TDP-43 overexpression in C. elegans. Hum Mol Genet 19: 3206-3218.
- Dosanjh LE, Brown MK, Rao G, Link CD, Luo Y (2010) Behavioral phenotyping of a transgenic Caenorhabditis elegans expressing neuronal amyloid-beta. J Alzheimers Dis 19: 681-690.
- Dostal V, Link CD (2010) Assaying beta-amyloid toxicity using a transgenic C. elegans model. J Vis Exp pii: 2252.
- Li A, Xie Z, Dong Y, Mckay KM, Mckee ML, et al. (2007) Isolation and characterization of the Drosophila ubiquilin ortholog dUbqln: in vivo interaction with early-onset Alzheimer disease genes. Hum Mol Genet 16: 2626-2639.
- Ganguly A, Feldman RM, Guo M (2008) Ubiquilin antagonizes presenilin and promotes neurodegeneration in Drosophila. Hum Mol Genet 17: 293-302.
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, et al. (1996) Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science 274: 99-102.
- Jacobsen JS, Wu CC, Redwine JM, Comery TA, Arias R, et al. (2006) Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A 103: 5161-5166.
- Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, et al. (2001) Age-dependent changes in brain, CSF, and plasma amyloid (beta) protein in the Tg2576 transgenic mouse model of Alzheimer's disease. J Neurosci 21: 372-81.
- Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, et al. (1999) Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice, Nat Neurosci 2: 271-276.
- Massaad CA, Washington TM, Pautler RG, Klann E (2009) Overexpression of SOD-2 reduces hippocampal superoxide and prevents memory deficits in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A 106: 13576-13581.
- Dutescu RM, Li QX, Crowston J, Masters CL, Baird PN, et al. (2009) Amyloid precursor protein processing and retinal pathology in mouse models of Alzheimer's disease. Graefes Arch Clin Exp Ophthalmol 247: 1213-1221.
- Liu B, Rasool S, Yang Z, Glabe CG, Schreiber SS, et al. (2009) Amyloid-peptide vaccinations reduce {beta}-amyloid plaques but exacerbate vascular deposition and inflammation in the retina of Alzheimer's transgenic mice. Am J Pathol 175: 2099-2110.
- Savonenko A, Xu GM, Melnikova T, Morton JL, Gonzales V, et al. (2005) Episodic-like memory deficits in the APPswe/PS1dE9 mouse model of Alzheimer's disease: relationships to beta-amyloid deposition and neurotransmitter abnormalities. Neurobiol Dis 18: 602-617.
- Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, et al. (2004) Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum Mol Genet 13: 159-170.
- Borchelt DR, Ratovitski T, Van Lare J, Lee MK, Gonzales V, et al. (1997) Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron 19: 939-945.
- Ning A, Cui J, To E, Ashe KH, Matsubara J (2008) Amyloid-beta deposits lead to retinal degeneration in a mouse model of Alzheimer disease. Invest Ophthalmol Vis Sci 49: 5136-5143.
- Thal LJ, Kantarci K, Reiman EM, Klunk WE, Weiner MW, et al. (2006) The role of biomarkers in clinical trials for Alzheimer disease. Alzheimer Dis Assoc Disord 20: 6-15.
- Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, et al. (2012) National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement 8: 1-13.
- Muchowski PJ, Ramsden R, Nguyen Q, Arnett EE, Greiling TM, et al. (2008) Noninvasive measurement of protein aggregation by mutant huntingtin fragments or alpha-synuclein in the lens. J Biol Chem 283: 6330-6336.
Relevant Topics
- Addiction Recovery
- Alcohol Addiction Treatment
- Alcohol Rehabilitation
- Amphetamine Addiction
- Amphetamine-Related Disorders
- Cocaine Addiction
- Cocaine-Related Disorders
- Computer Addiction Research
- Drug Addiction Treatment
- Drug Rehabilitation
- Facts About Alcoholism
- Food Addiction Research
- Heroin Addiction Treatment
- Holistic Addiction Treatment
- Hospital-Addiction Syndrome
- Morphine Addiction
- Munchausen Syndrome
- Neonatal Abstinence Syndrome
- Nutritional Suitability
- Opioid-Related Disorders
- Relapse prevention
- Substance-Related Disorders
Recommended Journals
Article Tools
Article Usage
- Total views: 14314
- [From(publication date):
specialissue-2013 - Dec 22, 2024] - Breakdown by view type
- HTML page views : 9848
- PDF downloads : 4466