Research Article Open Access
Alleviation of Lead Toxicity by Silicon is Related to Elevated Photosynthesis, Antioxidant Enzymes Suppressed Lead Uptake and Oxidative Stress in Cotton
| Bharwana SA1, Ali S1*, Farooq MA1, Iqbal N2, Abbas F1 and Ahmad MSA3 | |
| 1Department of Environmental Sciences, Government College University, Allama Iqbal Road, 38000, Faisalabad, Pakistan | |
| 2Department of Botany, Government College University, Allama Iqbal Road, 38000, Faisalabad, Pakistan | |
| 3Department of Botany, University of Agriculture, Faisalabad, Pakistan | |
| Corresponding Author : | Ali S Department of Environmental Sciences Government College University Allama Iqbal Road, 38000 Faisalabad, Pakistan Tel: +92 41 9201566 Fax: +92 41 9200671 E-mail: shafaqataligill@yahoo.com |
| Received April 05, 2013; Accepted May 09, 2013; Published May 11, 2013 | |
| Citation: Bharwana SA, Ali S, Farooq MA, Iqbal N, Abbas F, et al. (2013) Alleviation of Lead Toxicity by Silicon is Related to Elevated Photosynthesis, Antioxidant Enzymes Suppressed Lead Uptake and Oxidative Stress in Cotton. J Bioremed Biodeg 4:187. doi: 10.4172/2155-6199.1000187 | |
| Copyright: © 2013 Bharwana SA, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Lead (Pb) is known to cause highly toxic direct and indirect effects on plants, animals and humans, like other metals of its group. The mitigating effects of exogenous Silicon (Si) application on Pb stressed cotton plants were investigated in this hydroponic study. The following growth parameters were observed chlorophyll contents, photosynthesis, growth, Pb uptake and accumulation, Malondialdehyde (MDA), Hydrogen Peroxide (H2O2) contents, Electrolyte Leakage (EL), soluble protein and the activities of major antioxidant enzymes (superoxide dismutase, SOD; guaiacol peroxidase, GPX; ascorbate peroxidase, APX and catalase, CAT). Six treatments with three replicates including control (basal nutrients comprising neither lead nor silicon), 1 mM silicon (Si), 50 μM Pb, 100 μM Pb, 50 μM Pb with 1 mM Si and 100 μM Pb with 1 mM Silicon (Si) were studied.
| Keywords |
| Anti-oxidant enzymes; Cotton; Growth; Lead; Silicon; Oxidative stress; Photosynthesis |
| Introduction |
| Being sessile organism, plants life continuously exposed to unfavorable conditions and stresses that cause effects on growth, development and productivity. Heavy metals being toxic substances cause great injury to plants by modifying the metabolic processes. Heavy metals can be reactive depending on their oxidation states, causing the toxicity to plant cells in many ways. Due to heavy metal toxicity different modifications occurs in plant physiological processes at both the cellular and molecular level, including inactivation of enzymes, functional groups blockage, replacement of certain metal ions of bio-molecules, conformational changes and the integrity of the membrane disruption, which finally give change in metabolism of plant, photosynthesis inhibition, respiration, and disruption in enzymatic activities [1]. |
| The pollution of agricultural lands due to heavy metals causes seriousproblems to the environment [2]. Many heavy metals have been found in various biotas, including soil, water and air [3] and lead (Pb) is one of the major concerns among heavy metals. Lead (Pb) caught more attention because of their long persistence in soil and cause extremely toxic effects on both the production of crops and on human health due to consumption of crops. Pb contamination originates mainly from mining and smelting processes, industrial wastes, agriculture (pesticides) and urban activities (additive in petroleum and paint) [4]. Lead (Pb) not only affects productivity and growth of plants but also cause health hazards to animals and human beings after entering into the food chain [5]. Unlike organic pollutants heavy metals cannot be degraded usually and their clean up from soil is a cumbersome task [6]. |
| It is well known that lead (Pb) toxicity in the environment cause significant toxic effects on plant processes including depression on seed germination [7], disorders in mitosis [8], induction of leaf chlorosis [9], root shoot growth reduction [10], enzyme activation and inhibition [11]. Different remediation approaches have been implicated to overcome the metal stress on crop plants. The use of silicon produces potential in crops to resist the stressful environment [12]. Last few years supplementation of silicon has got considerable consideration to improve the tolerance ability in crop against the heavy metal stress. |
| Silicon (Si) is the second most abundant element both on the surface of the earth and in the soil [13]. Silicon (Si) in soil is insoluble and not available to plants because of their combination with other compounds to form silicates or oxides [14]. Usually, Silicon (Si) is absorbed by plants in the form of uncharged silicic acid, Si(OH)4 [14] and is ultimately polymerized to form silica gel (SiO2.nH2O). Silicon (Si) concentrations vary with plant species and tissues (on dry weight basis) is in a range of 0.1 to 10.0% [15]. Although silicon is not evidenced as anessential element for higher plants, but silicon has been demonstrated to have multiple direct and indirect positive effects on growth and development of many plants [16-18]. The positive role of Silicon (Si) is obviousin plants when exposed to biotic and abiotic stresses [17-19]. It has been well documented that silicon enhances the tolerance of plants to metals toxicity, including Aluminum (Al) [20-22] Boron (B) [23,24], Cd [25-27] Manganese (Mn) [28-30] and Zinc (Zn) [31-33]. |
| On the whole, Silicon (Si) is recommended to be used for enhancing the tolerance of plants to abiotic stresses, including heavy metals toxicity. However, the possible effect of Silicon (Si) in alleviating Pb toxicity has not been reported up to date in cotton plants. In this study, hydroponic experiment was conducted to investigate the impact of Si on plant growth, photosynthesis, chlorophyll contents, oxidative stress, antioxidant enzymes, electrolyte leakage and lead (Pb) uptake under Pb stress, so as to determine whether exogenous Silicon (Si) may activate protective responses of cotton plants exposed to lead stress and understand the possible mechanisms of alleviating lead toxicity by Silicon (Si) application. |
| Materials and Methods |
| Experimental site |
| This study was carried out in wire house of Ayub Agricultural Research Institute (AARI) and, in labs of Government College University Faisalabad and Nuclear Institute for Agriculture and Biology (NIAB) Faisalabad, Pakistan. |
| Growth conditions |
| Healthy seeds of cotton genotype MNH 886 was immersed in concentrated sulfuric acid solution for 15 min to remove the short fiber on the surface of the seed. Seeds were then rinsed with distilled water thoroughly and sown in two-inch layers of sterilized quartz sand trays in a growth chamber with a photoperiod of 16 h light/ 8 h dark with light intensity of 400 ± 25 μmolm−2s−1. The light/dark temperature was set at 30°C/25°C with relative humidity at 85%. After two weeks of sowing, the uniform seedlings were wrapped with foam at a root shoot junction, and transplanted in thermopole sheets having evenly spaced holes floating on 40 L capacity iron tubs, lined with polyethylene sheet containing modified Hoagland’s solution. The basic nutrient medium had composition: (Ca(NO3)2 2.5 mM, MgSO4 1mM, KCL 0.5mM, KH2PO4 0.5mM, FeCl3 0.1μM, CuSO4 0.2μM, ZnSO41μM, H3BO3 20μM, H2MoO4 0.005μM, MnSO4 2μM). Continuous aeration was given through an air pump in the nutrient solution by making bubbles. The solution was changed every week. Complete Randomized Design (CRD) was applied. Two weeks after transplanting, lead (Pb) levels (control, 100 and 50 μM) developed with Pb(NO3)2 and two levels of Silicon (Si) as sodium silicate (Na2SiO3)(control and 1mM) with three replicates were applied. By adding 1M H2SO4 and NaOH solution pH (6.0 ± 0.1) was maintained. |
| Measurements |
| Plants were harvested after six weeks of growth under lead (Pb) stress. Data regarding shoot, root length, shoot, root fresh and dry weight were determined. |
| Leaf area |
| Leaf area was determined with a leaf area meter (LI-2000, LI-COR, USA). |
| Gas exchange parameters |
| Six weeks after application of treatment photosynthetic rate (A), stomatal conductance (gs), transpiration rate (E), water use efficiency (A/E) was determined by using Infra-Red Gas Analyzer (IRGA) (Analytical Development Company, Hoddesdon, England). A week later SPAD value was measured by the SPAD-502 meter. |
| Determination of photosynthetic pigments |
| Chlorophyll a, chlorophyll b, total chlorophyll and carotenoids were determined by spectrophotometrically. After six weeks of treatment, the topmost fully expanded leaves were taken for the extraction of the chlorophyll pigments. Freshly weighed leaves dip in 85% (v/v) aqueous acetone for the extraction of the chlorophyll pigments. Absorbanceat 452.5, 644 and 663 nmwas determined using a spectrophotometer (AA6300, Shimadzu, Kyoto, Japan). |
| Chl a, b, total chlorophyll and carotenoids were calculated according to Lichtenthaler [34]. |
| Estimation of electrolyte leakage |
| After treatment of six weeks, leaves samples were cut into small parts of 5 mm length. Electrolyte leakage was estimated by using the method of Dionisio-sese and Tobita [35] |
| Assay of antioxidant enzymes |
| Anti-oxidant enzymes such as Superoxide Dismutase (SOD), Guaiacol Peroxidase (POD), Catalase (CAT), and Ascorbate Peroxidase (APX) in roots and leaves were determined by spectrophotometrically. |
| After six weeks of treatment fresh samples (0.5g) of leaves and roots were ground with the help of a motor and pestle and homogenized in 0.05 M phosphate buffer (pH 7.8) under chilled condition. The homogenized mixture was filtered through four layers of muslin cloth and centrifuged at 12,000×g for 10min at 4 °C. Supernatant was taken and Zhang [36] method used for the measurement of SOD, POD activities. |
| Catalase (CAT, EC 1.11.1.6) activity was determined by the method of Aebi [37]. The CAT activity was assayed by monitoring the decrease in the absorbance at 240 nm as a consequence of H2O2 disappearance. |
| Ascorbate peroxidase (APX, EC 1.11.1.11) activity was assayed according to the method of Nakano and Asada [38]. The oxidation of ascorbate was determined by the change in absorbance at 290 nm. |
| Determination of soluble protein |
| The soluble protein content was analyzed according to Bradford [39], using Coomassie Brilliant Blue G-250 as dye and albumin as a standard. |
| Hydrogen peroxide contents |
| H2O2 was extracted by homogenizing 50 mg leaf or root tissues with 3ml of phosphate buffer (50 mM, pH 6.5). Then, the homogenate was centrifuged at 6,000×g for 25 min. |
| To measure H2O2 content, 3 ml of extracted solution was mixed with 1 ml of 0.1% titanium sulphate in 20% (v/v) H2SO4 and the mixture was then centrifuged at 6,000 g for 15 min. The intensity of the yellow color of the supernatant was measured at 410 nm. H2O2 content was computed by using the extinction coefficient of 0.28 μmol-1cm-1. |
| Malondialdehyde (MDA) Content |
| The level of lipid peroxidation in the leaf tissue was measured in terms of malondialdehyde (MDA, a product of lipid peroxidation) content determined by the Thiobarbituric Acid (TBA) reaction using the method of Heath and Packer [40], with minor modifications as described by Dhindsa et al. [41] and Zhang and Kirham [42]. |
| Estimation of lead (Pb) concentration |
| After six weeks of treatment, cotton plants were harvested with each treatment having three replications, and washed with tap water, distilled water and deionized water thoroughly. After that, plant samples were separated into roots, stem, and leaves dried at 80°C in an oven for 48 hours, and then ground into powder. 0.5 gram of each sample was dry-ashed, extracted with HCl and then centrifuged at 3600 rpm (Revolutions per minute) for 15 min. Concentrations of Pb in root, stem and leaves were determined by a flame atomic absorption spectrometry. |
| Statistical analysis |
| All values reported in this experiment are means of three replicates. Analysis of variance (ANOVA) was carried out by using a statistical package, SPSS version 16.0 (SPSS, Chicago, IL) followed by Tukey test between means of treatments to determine the significant difference. |
| Results |
| Plant growth parameters |
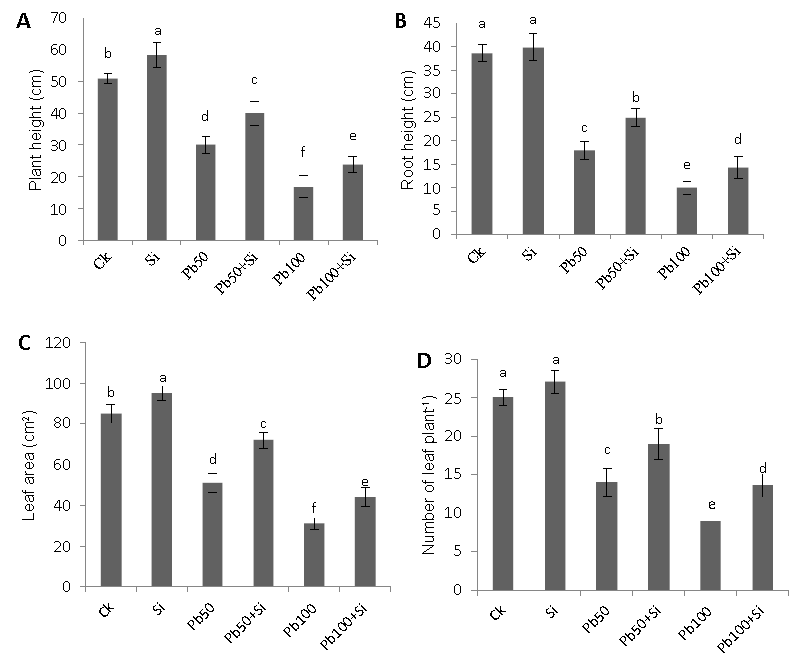
| After six weeks of treatment with lead (Pb) toxicity significant effect were observed on plant growth. The effect of exogenous silicon on the plant growth under lead stress in terms of plant height, root length, number of leaves plant-1 and leaf area are presented in figure 1. When we added Si alone to culture solution cotton growth was slightly increased as compared to the control. Addition of Pb to the solution caused a dramatic and significant reduction in all four cotton growth parameters on both lead levels viz. 50 μM and 100 μM as compared to control. Moreover the decrease was dose dependent. Growth inhibition upon exposure to lead stress was dramatically alleviated by Si addition in both lead stress levels (50 μM and 100 μM), reflected by increment in plant height, root length, number of leaves plant-1 and leaf area. It may be suggested that Si had alleviating role in plants with Pb stress conditions. At the end of the experiment, the appearance of the plants differed greatly between the treatments; control plants grown in the absence of Si (control) were shorter than those grown with Sitreatment. Similarly, the plants of the lead treatment in both lead levels (50 μM and 100 μM) were smaller than the plants grown with Pb+Si. The positive effect of Si was greater for plants treated with lead as compared to control plants. |
| Plant biomass |
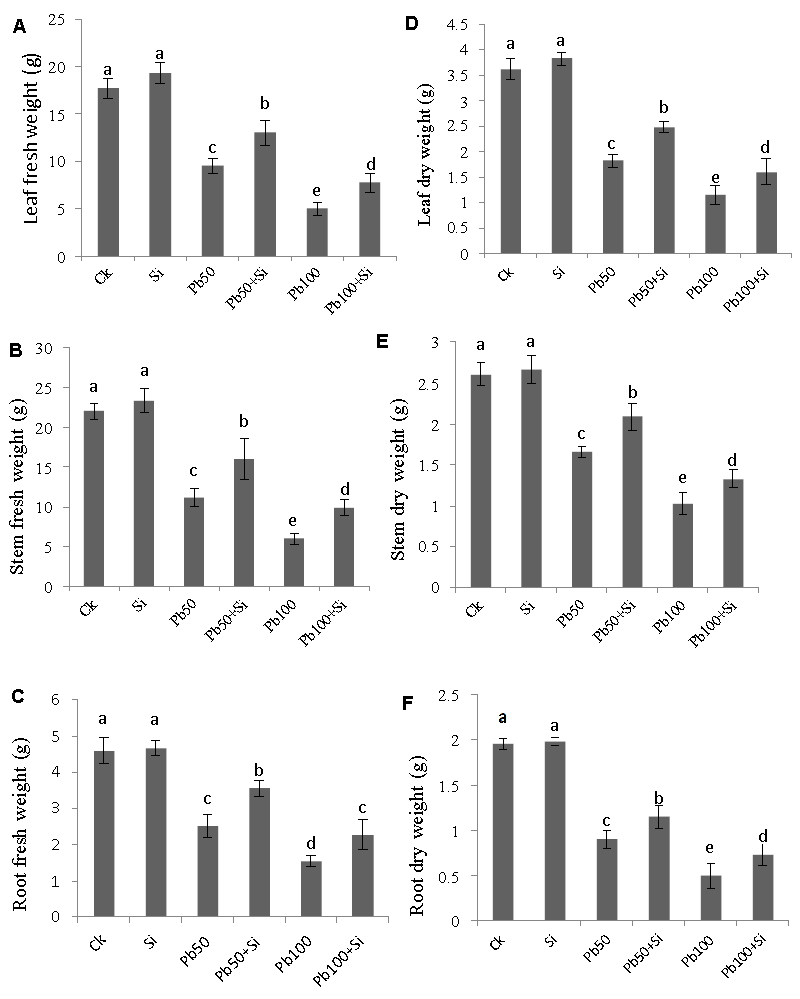
| The effect of exogenous silicon on cotton plant’s biomass was examined in terms of leaf fresh weight, stem fresh weight, root fresh weight, leaf dry weight, stem dry weight and root dry weight under lead stress is shown in figure 2. Lead stress clearly inhibited the leaf fresh weight, stem fresh weight, root fresh weight, leaf dry weight, stem dry weight and root dry weight on both stress levels (50 μM and 100 μM) and the inhibitory effect was more prominent at 100 μM level being significantly different at 5% probability level from the respective controls. Silicon addition helped the plants to tolerate lead stress conditions. The plant biomass under both stress levels (50 μM and 100 μM) was recovered by Si application (1 mM) remarkably. |
| Photosynthetic pigments and SPAD value |
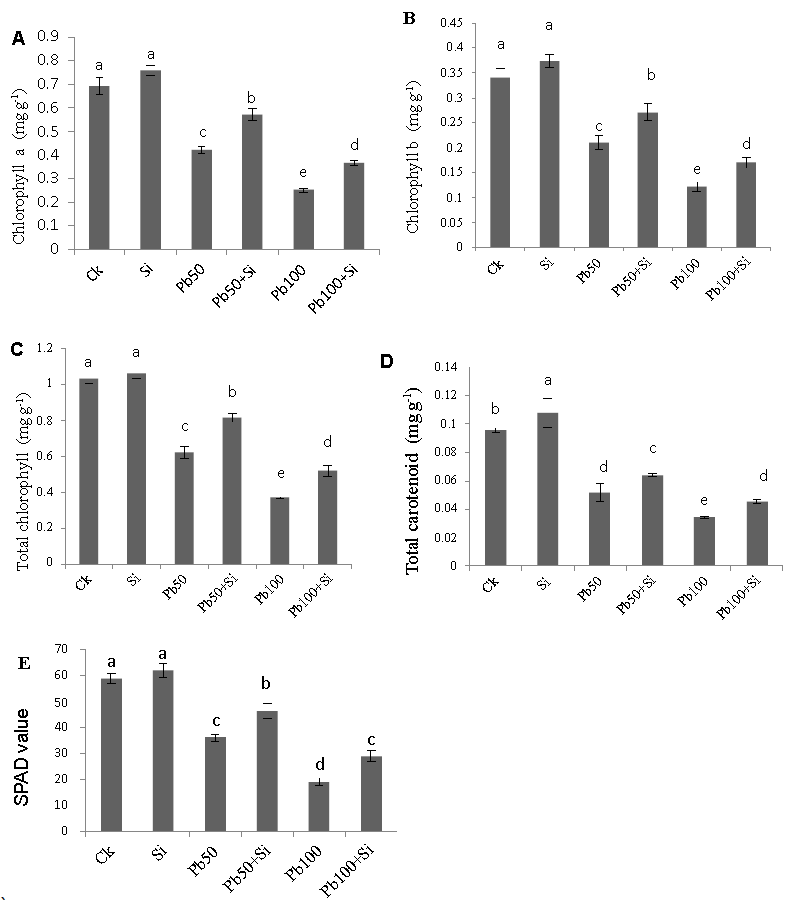
| The effect of three different levels of lead stress (0, 50 μM and 100 μM) and two levels of Si (0 and 1 mM) on photosynthetic pigments such as chlorophyll a, chlorophyll b, total chlorophyll, carotenoids and SPAD value are shown in figure 3. Pb stress caused a significant decrease in photosynthetic pigments such as chlorophyll a, chlorophyll b and total chlorophyll, carotenoids and SPAD value at both lead levels (50 μM and 100 μM) in cotton plants relative to the control. In the absence of lead, silicon addition alone (1mM) in the nutrient solution led to slight increase in all these observed parameters as compared to control. However, silicon showed significant effect in alleviating the reduction of chlorophyll a, chlorophyll b, total chlorophyll, carotenoids and SPAD value caused by lead stress at both Pb levels (50 μM and 100 μM). |
| Gas exchange parameters and water use efficiency |
| Figure 4 show the changes induced by lead and silicon either alone or in combination in cotton plants on gas exchange parameters expressed as net photosynthetic rate (Pn), stomatal conductance (Gs), transpiration rate (E) and water use efficiency (Pn/E). All the photosynthetic parameters viz. net photosynthetic rate (E), stomatal conductance (gs), transpiration rate (E) and water use efficiency (Pn/E) was declined by the lead stress treatments. The inhibitory effects on all these parameters were more pronounced and marked at 100 μM compared to the control plants. Exogenous application of silicon appeared to alleviate the inhibitory effect induced by lead stress. The maximum values of all these gas exchange parameters were observed in the plants treated with silicon alone. |
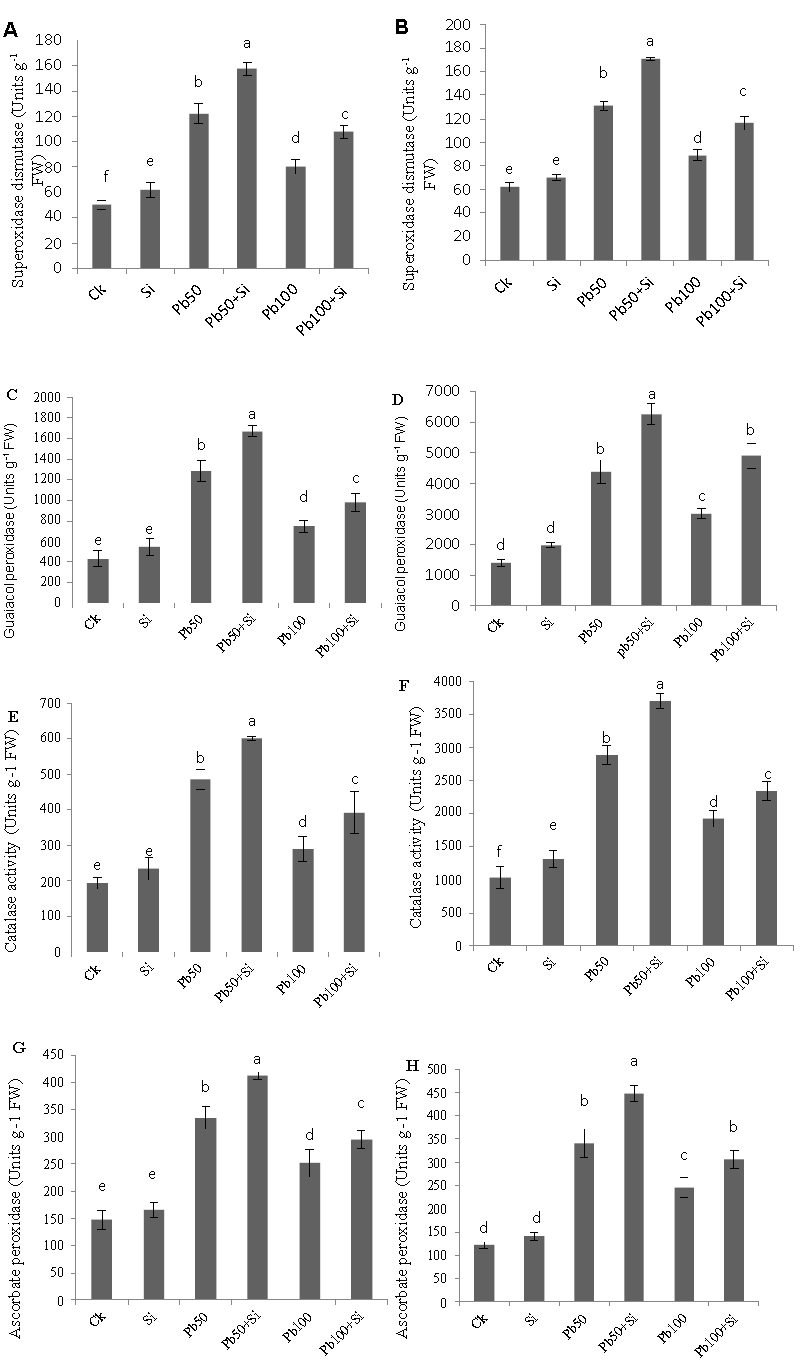
| Activities of anti-oxidative enzymes |
| Figure 5 highlighted the effect of lead stress and silicon alone or in combination on four different antioxidant enzymes such as SOD, POD, CAT and APX in both leaves and roots. APX and POD are the two major key enzymes for the removal of H2O2 from the chloroplasts. CAT is mainly responsible for eliminating H2O2 from the peroxisomes and SOD for catalyzing the dismutation of O2 to O2 and H2O2. Control cotton plants showed the minimum values of antioxidant enzymes. Under lead stress at 50 μM, all four tested antioxidants increased significantly as compared to control but again a decrease was observed in all antioxidant enzymes tested. Moreover, exogenous application of silicon to the lead-treated plants had synergetic effect on the anti-oxidant enzyme activities on both lead levels (50 μM and 100 μM). |
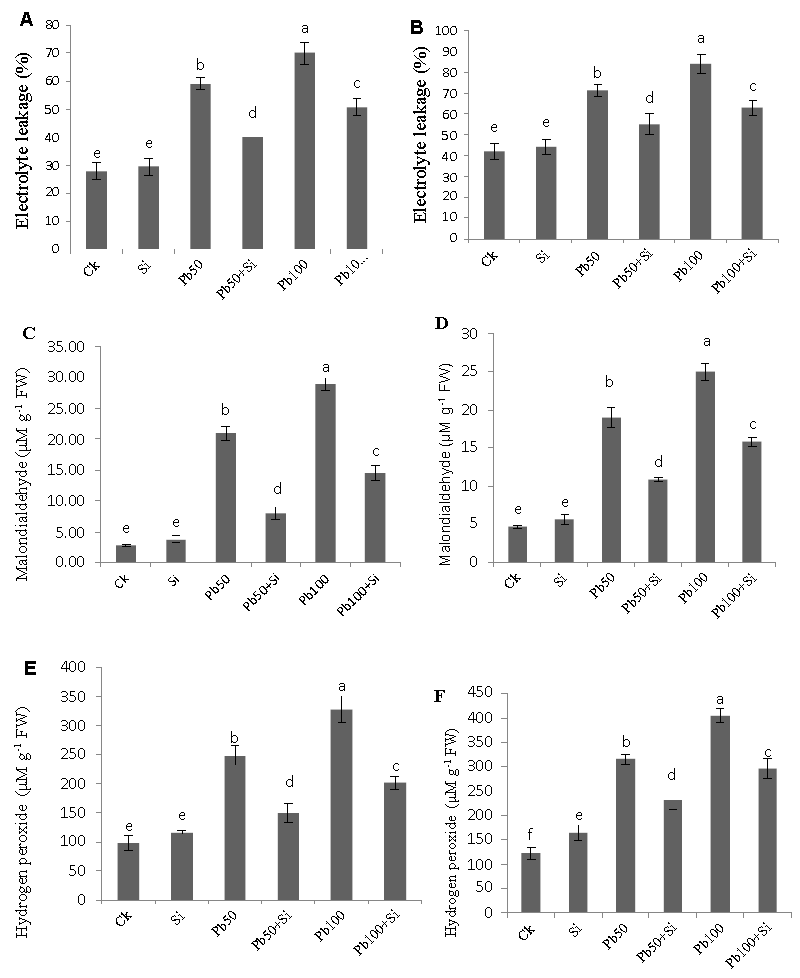
| Electrolyte leakage, malondialdehyde and hydrogen peroxide |
| Effect of three different concentrations of lead stress and two concentrations of silicon, alone and in combination was investigated on the Electrolyte leakage (EL), malondialdehyde (MDA) and hydrogen peroxide (H2O2) in cotton plants both in leaves and roots (Figures 6). Degree of cell membrane injury was measured indirectly by solute leakage of lead treated plants in the absence or presence of silicon. Lead stress induced a significant increase in the membrane injury and increased electrolyte leakage significantly. Silicon application decreased EL significantly in both lead levels (50 μM and 100 μM). MDA contents in cotton plants exposed to stress and adverse environmental conditions are an authentic indicator of free radical formation in the plant tissues. MDA content in both roots and leaf increased as the lead level rose as compared to control. The exogenous application of silicon remarkably decreased the MDA content in the stressed cotton seedlings at both lead levels (50 μM and 100 μM). It was also observed that lead induced stress while silicon application alone or in combination with lead reduced the level of H2O2 in both leaves and roots. Treatment with 100 μM lead alone induced the highest concentration of H2O2 whereas silicon alone showed a lowest concentration as compared to their respective controls. Exogenous application of silicon to the plants treated with both lead levels helped plants to adjust the level of H2O2. |
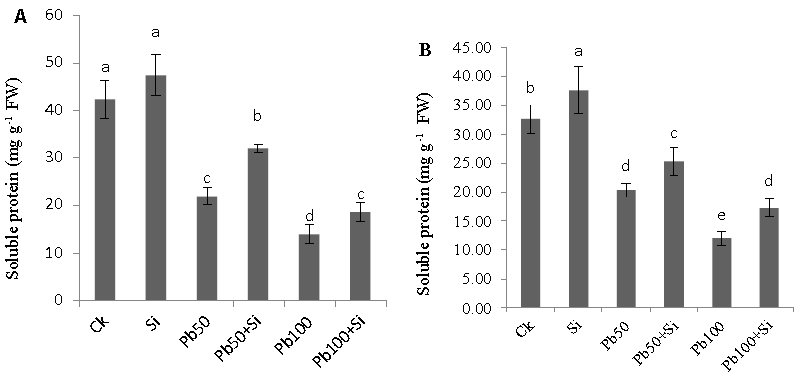
| Soluble protein |
| Soluble protein contents in leaves and roots of cotton plants are shown in figure 7. soluble protein contents in both roots and leaves of the cotton plants were significantly reduced by the addition of lead on both levels (50 μM and 100 μM) in nutrient solution. However, the reduction was significantly alleviated by the exogenous application of silicon at both levels. Moreover, in absence of lead, exogenous silicon application also increased the soluble protein contents in both leaves and roots as compared with the control. |
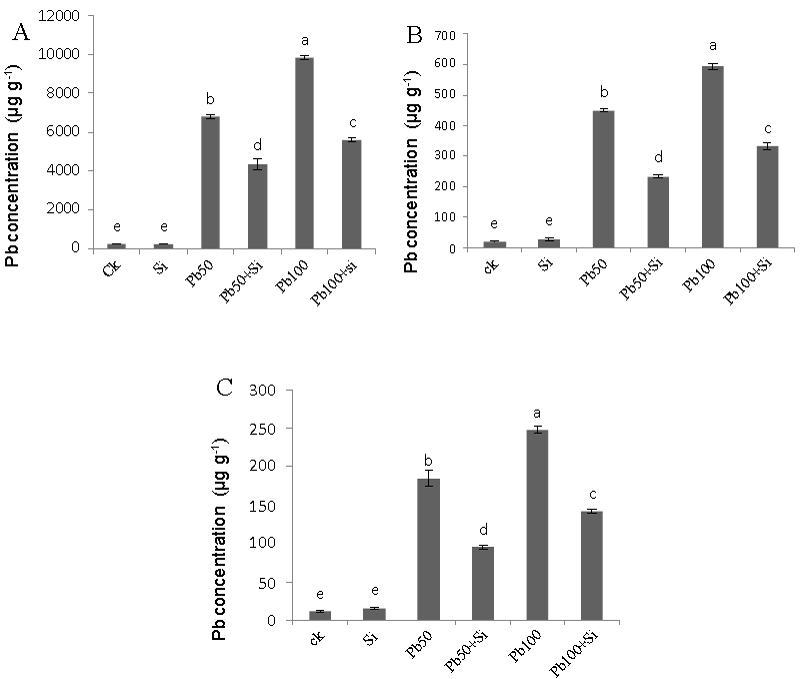
| Lead contents |
| Lead (Pb) contents in roots stem and leaves are presented in figure 8. Lead was mainly deposited in the below-ground parts of lead treated cotton plants. The addition of lead in nutrient solution at both levels (50 μM and 100 μM) increased the Pb uptake as lead levels increased. The degree of increase in uptake in all three plant parts viz. root, stem and leaves was dependent on dose. Roots had much higher Pb contents irrespective of lead levels followed by stem and leaves. Silicon addition significantly reduced lead concentration in all three plant parts at both lead levels. Moreover, silicon addition resulted in a significant decrease of lead translocation from roots to aboveground parts of the plants treated with lead at both levels (50 μM and 100 μM). |
| Discussion |
| In recent years, data has been accumulated that indicates the beneficial effects of silicon on the growth and development of many plant species. Present study was done because the effects of exogenous silicon addition on lead (Pb) tolerance in plants are unclear and still lacking. Present results showed that Pb treatment markedly decreased plant growth and biomass. It is already observed in many studies [43-46], that lead affected plant growth and development due to the biochemical and metabolic processes. Moreover, in our present investigation Pb inhibited gas exchange parameters such as net photosynthetic rate (Pn), stomatal conductance (gs), transpiration rate (E) and water use efficiency (Pn/E) and chlorophyll a, chlorophyll b, and total chlorophyll, carotenoids and SPAD value. Similar results were observed by the previous researchers [47,48]. |
| The present study showed that plant growth, biomass, chlorophyll contents and photosynthetic parameters of cotton plants were enhanced by silicon addition under non-Pb stress condition, proving the positive and beneficial effects of silicon (Si) on cotton growth. The reason for this beneficial effect of Si is still unclear. Similarly, many researchers found beneficial effects of silicon in many crops such as cotton, rice and sugarcane [18-21] Such beneficial impacts could be indirect (for instance improved disease resistance) or directly improved nutrition. It was hypothesized by Strubinskai et al. [44], that silicon induced promotion in growth of rice plants which resulted an increase in cell wall extensibility. |
| The results of present investigation showed that silicon markedly alleviated lead- induced reduction in growth, biomass and photosynthetic parameters in cotton plants. The alleviating effects of silicon on stresses like high temperature, chilling, diseases, drought, salinity, heavy metals etc. have been intensively studied. To our knowledge, it was the first ever investigation on the interaction of lead (Pb) and silicon in plants. It was reported by many scientists that silicon supplement would enhance tolerance to toxic metals through reducing the uptake and translocation of them, including Cd, Mn and Zn [49,50]. Our present results also showed that lead (Pb) decreased soluble protein in both roots and leaves of cotton plants. It may be due more oxidative damage might be occurred that inhibited the protein contents [51]. In the present investigation, increased Reactive Oxygen Species (ROS) was found under metal stress as indicated by electrolyte leakage, MDA and H2O2 contents. Plants generally face the oxidative damage when exposed to lead and other metals [33,52,53]. To meet and check the oxidative damage in plants developed complex antioxidant system. In the present study anti-oxidant enzymes such as SOD, POD, CAT and APX both in roots and leaves first increased up to 50 μM but again decreased at higher lead level (100 μM). The decrease in anti-oxidant enzymes at higher lead concentrations which might be due to the severe stress of oxidative damage to antioxidant enzymes, hence, the activity of enzymes was diminished [54]. Alleviation of metals toxic effects by silicon (Si) was also related with protection against oxidative damage due to oxidative stress. silicon (Si) alleviated B stress in spinach, barley and tomato by preventing oxidative damage [27]. Similarly, MacFarlane [55] reported that the alleviation of cadmium toxicity by Si in pakochi plants was related to a marked and significant increase in enzymatic and non-enzymatic antioxidants and decrease in membrane lipid peroxidation (MDA) [54]. According to Mishra S [56], Si-enhanced tolerance to Mn toxicity in cucumber plants was attributed to Si. In the present investigation, exogenous addition of silicon in solution culture showed marked beneficial effects on MDA, H2O2 and electrolyte leakage anti-oxidant activities of SOD, APX, POD and CAT under lead stress. From our results, it might be suggested that silicon addition could markedly enhance the capacity of defense against oxidative damage induced by lead toxicity in cotton seedlings. |
| The lead contents in all three parts of cotton plants in present results increased as we increased the Pb concentration in culture solution. In this investigation, lead was less translocated from roots to shoots in cotton plants. Mostly, the plants with highest resistance take up less proportion of the total metal solution and had the lowest upper ground metal contents [57]. But, exogenous application of Si decreased the uptake of lead(Pb) in all three parts of plants such as roots, stem and leaves and translocation of lead (Pb) in Pb stressed plants as compared to Pb alone. The possible mechanisms for inhibition of metal transport in plants by Si are mainly involved in the two aspects: (1) Si deposits lignin in cell walls and induces metal ions bound to cell walls, thus reduce translocation of metals from roots to shoots [16]. It was reported that Mn stress tolerance enhancement in Cucumis sativus by silicon (Si) was a result of stronger binding of manganese to cell walls and a decreasing of Manganese contents within symplast [3] Similarly, it was observed that other metals such as Cd and Al behaved like same [58,21] (2) the complex formation or co-precipitation of toxic metal ions with Si. Liu J et al. [59] reported that silicon could detoxify Al toxicity in Sorghum bicolor through a complex formation with Al in the medium and/or roots and ultimately inhibiting Al penetration into the root cortex. Similarly, Wang L et al. [60] reported that formation of B-Si (boron-silicate) complexes in the soil led to lower boron availability and consequently lowered tissue boron concentration. In the present study, Si-induced inhibition of lead (Pb) uptake and translocation in cotton plants was also observed. Therefore, it can be supposed that silicon (Si) might also have the ability to complex with lead (Pb) or induce Pb to deposit in the cell walls, thus lowering lead (Pb) toxicity in cotton plants. |
| In conclusion, Silicon (Si) is very important and beneficial for growth and development of many plant species exposed to different types of abiotic and biotic stresses. Present results revealed that lead caused significant decrease in cotton growth, biomass, pigments, photosynthetic characteristics, protein by enhancing lead (Pb) uptake and accumulation, MDA, H2O2 and electrolyte leakage and by lowering antioxidant capacity. But, silicon addition in nutrient solution protects cotton seedlings against lead stress by lowering uptake and accumulation of lead, MDA, H2O2 and electrolyte leakage and by enhancing antioxidant enzymes. It can be expected that silicon addition in lead contaminated soils can help to lowering lead (Pb) contamination of grains by decreasing lead (Pb) accumulation in grains. |
| Acknowledgements |
| Thanks to Higher Education Commission of Pakistan for financial support. The results presented in this paper are a part of PhD studies of Ms Saima Aslam Bharwana. |
References
|
Tables and Figures at a glance
 |
 |
 |
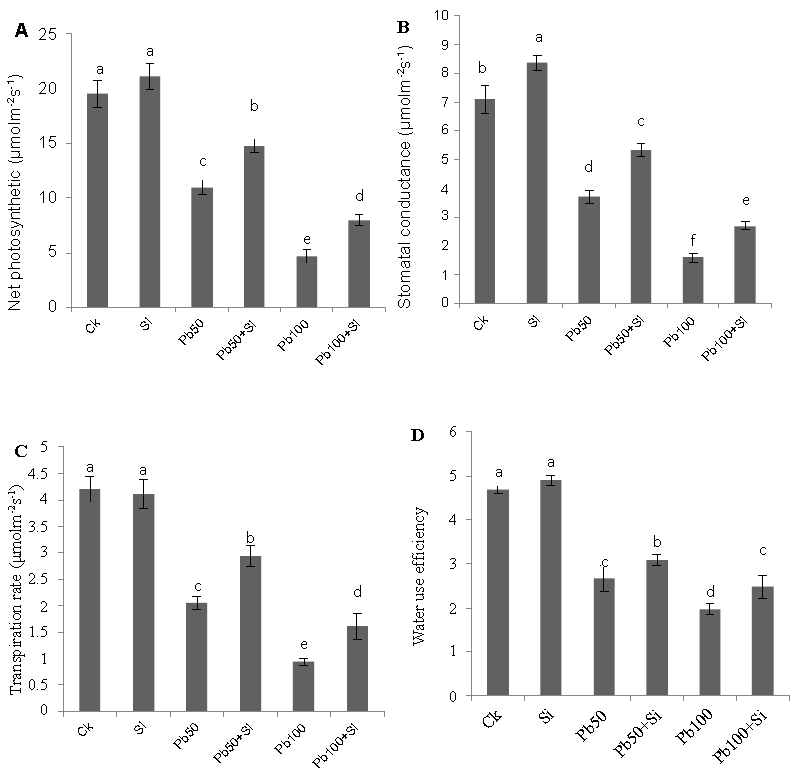 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
 |
 |
 |
 |
| Figure 5 | Figure 6 | Figure 7 | Figure 8 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 16633
- [From(publication date):
May-2013 - Jul 18, 2024] - Breakdown by view type
- HTML page views : 11725
- PDF downloads : 4908
