Research Article Open Access
Alkane Degradative Potentials of Bacteria Isolated From the Deep Atlantic Ocean of the Gulf of Guinea
| Sunday Babatunde Akinde1*, Catherine Chinenye Iwuozor2 and Omokaro Obire3 | |
| 1Department of Biological Sciences, College of Science, Engineering and Technology, Osun State University, P.M.B. 4494, Osogbo, Nigeria | |
| 2Fugro Nigeria Limited, 91 Odani Road (Fugro Avenue), Elelenwo, P. M. B. 053, Port Harcourt, Nigeria | |
| 3Department of Applied and Environmental Biology, Rivers State University of Science and Technology, Nkpolu, P.M.B. 5080, Port Harcourt, Nigeria | |
| Corresponding Author : | Akinde Sunday Babatunde Department of Biological Sciences College of Science, Engineering and Technology Osun State University, P.M.B. 4494, Osogbo, Nigeria Tel: +234 8033798688 E-mail: akindesb@uniosun.edu.ng |
| Received December 12, 2011; Accepted January 20, 2012; Published January 22, 2012 | |
| Citation: Akinde SB, Iwuozor CC,Obire O (2012) Alkane Degradative Potentials of Bacteria Isolated From the Deep Atlantic Ocean of the Gulf of Guinea. J Bioremed Biodegrad 3:135. doi: 10.4172/2155-6199.1000135 | |
| Copyright: © 2012 Akinde SB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
The efficiency of bacterial strains isolated from Nigeria Deep Atlantic Ocean water and sediment (2450 m – 2774 m depths) to biodegrade total petroleum hydrocarbons (TPH, n-alkanes) constituents of 1% crude oil was compared with that of similar isolate from oily wastewater reservoir in Imo State, Nigeria. Standardized bacterial strains (OD546 1.0) with similar morphological and biochemical characteristics from surface seawater (HBW12a), bottom seawater (HBW12b), superficial sediment (HBS12) and oily wastewater reservoir (HMWP) were selected and their TPH biodegradation efficiency in 30 days was quantified by gas chromatographic analysis (GC-FID). TPH fractions (n-C8–n-C40) of Bonny light crude oil was degraded significantly (P≤0.05) by all the four isolates. HMWP, HBW12b, HBS12 and HBW12a degraded 85.7%, 80.8%, 79.1% and 78.6% of TPH respectively. Although bacterial isolates from oily wastewater reservoir (HMWP) possessed highest degradative capabilities, isolates from seawater surface (HBW12a) and seawater bottom (HBW12b) as well as sediment (HBS12) also exhibited crude oil degradative potential. The four isolates have been tentatively identified as Pseudomonas. The gas chromatograms showed a distribution from n-C11 to n-C36 alkanes with the fractions of n-C15, n-C17 and n-C19 showing relatively high concentrations at the onset of experiment followed by a significant degradation in 30 days by all the isolates. The n-C17/pristane peak ratios of 0.43, 0.47, 0.47 and 0.48 for HMWP, HBW12a, HBW12b and HBS12 respectively in day 30 also affirmed TPH biodegradation by all the isolates. Indigenous bacteria from deep Atlantic Ocean water column and sediment thus possess capabilities for biotechnological applications in deep water bioremediation.
| Keywords |
| Total petroleum hydrocarbon; Alkane-degrading bacteria; Hydrocarbon utilizing bacteria; Biodegradation; Deep Atlantic Ocean; Gulf of Guinea; Bacteria; Pseudomonas; Seawater |
| Introduction |
| Man’s inexorable demand for petroleum products has intensified the search for crude oil and gas in regions of the world, which hitherto were unexplored. This has led to the expansion of petroleum developments in Nigeria into deep offshore areas including deep Atlantic Ocean of the Gulf of Guinea. |
| Gulf of Guinea deep seawater starts from 200 metres water depth just immediately after the continental shelf. Exploration efforts started in this zone in 1993 with the award of 18 blocks to 12 concessionaries [1]. The first commercial Nigeria deep seawater discovery (Bonga) was made in 1996 in water depths of about 1,000 m [2]. Since then, several other deep offshore discoveries have been made and oil production activities are currently taking place in the Nigeria segment of the deep Atlantic Ocean of the Gulf of Guinea up to 3,000 m water depths. |
| Oil producing areas of Nigeria especially the Niger-Delta area have experienced the devastating consequences of crude oil spills to both terrestrial and aquatic environments in the past 50 years of crude oil exploration and production. One of the major reasons for prolonged negative impact of oil spill on the environment could probably be due to absence of adequate and qualitative scientific baseline data which is required to provide informed and quick response to emergent environmental challenges. Urgent and high research efforts are therefore required to acquire basic scientific knowledge especially on the biotic activities of the deep Atlantic Ocean of the Gulf of Guinea for sustainability. |
| During the life span of crude oil exploration and production activities in the deep seawater environment, occasional oil spillages are inevitable. In the wake of 20th December 2011, oil spillage which occurred in the process of loading a crude vessel in Bonga Floating Production Storage and Offloading (FPSO) in deep offshore Nigeria was announced [3]. While the oil and gas industries are genuinely concerned with preventing oil spills, their capabilities to contain and clean up spilled oil in deep Atlantic Ocean of the Gulf of Guinea and bioremediate the seawater environment have not been proven to be effective under real conditions. Consequently, crude oil components which enter this environment will be left for natural microbial populations to degrade hence the need to test the crude oil degradative capabilities of these indigenous microbiota. |
| Several studies have already been carried out to characterize heterotrophic bacteria in ocean sites and in different coastal areas of temperate, tropical and polar zones [4-9]. Data have been published describing the distribution of bacterial densities which depend on changes in water temperature, salinity, the abundance of organic nutrients and on other physico-chemical parameters [9-12]. However, it has been recognized that bacterial populations may be considerably modified by interactions with other biotic factors [13]. In an over-simplified way, the density of heterotrophic bacterial populations in sea water usually ranges from 103 to 106 ml-1, with counts up to 109 g-1 recorded for sediments. In the deep seawater column, the presence of heterotrophic bacteria usually decreases with increasing depth [9]. |
| In the marine environment, 90% of heterotrophic bacteria are Gram negative rods with different characteristics and the Gram negative cell wall is better adapted for survival in the marine environment [14]. The majority of the isolates belong to the genera Pseudomonas, Vibrio and Flavobacterium. Akinde and Obire [9] had earlier isolated Gram negative heterotrophic bacteria from the deep Atlantic Ocean of the Gulf of Guinea which showed taxonomic affinities to the genus Pseudomonas. |
| Heterotrophic microorganisms are the major agents shaping the organic composition of the Ocean [15]. These heterotrophic bacteria comprise the bulk of microbial populations inhabiting the water column of oceans and are responsible for much of the biological transformation of organic matter and production of carbon dioxide [16]. Distribution of bacteria depends on changes in water temperature, salinity and other physicochemical parameters [17]. Bacteria also serve as an important source of food for a variety of marine organisms. Thus, bacteria not only maintain the pristine nature of the environment, but also serve as biological mediators through their involvement in the biogeochemical processes [14]. |
| Crude oil is an extremely complex mixture of hydrocarbons. From the hundreds of individual components, several classes, based on related structures can be recognized. Various classes include the saturate or aliphatic fraction, the aromatic fraction and the asphaltic or polar fraction [18]. The mechanism for degradation of crude oil hydrocarbons has been studied extensively [19,20] and a number of microorganisms, including bacteria, fungi and yeasts, have been isolated and characterized for their ability to degrade crude oil hydrocarbons. Alkane-degrading bacteria have been isolated from deep-sea Mediterranean sediments [21]. However, there is no information regarding the activities of this group of bacteria in the deep Atlantic Ocean of the Gulf of Guinea. |
| The objectives of the study therefore is to observe the potentials of hydrocarbon utilizing heterotrophic bacterial (HUB) isolates of deep Atlantic Ocean to biodegrade total petroleum hydrocarbon (TPH) or alkane components of Bonny light crude oil using optimal conditions to provide selected organisms which can be used in hastening the biotreatment process of a crude oil polluted deep seawater body. |
| Materials and Methods |
| Study area is the deep Atlantic Ocean of the Gulf of Guinea, located at approximately 150 – 170 km from the nearest Nigerian shoreline and within seawater depths of 2450 – 2774 m. It lies within geographic coordinates 3.5674611° – 4.01808417°E and 4.08667528° – 4.43155139°N, offshore Nigeria. Crude oil exploration activities have not started in this concession as at the time of sample collection. |
| Bonny light crude oil was obtained from the Bonny Terminal of Shell Petroleum Development Company of Nigeria. It has 81.1%, 7.20%, 2.48% and 0.21% of saturation, aromatics, asphaltenes and residues respectively [22]. |
| During one of the RV Geoexplorer cruises in the Nigeria segment of the deep Atlantic Ocean, surface and bottom seawater samples were obtained with Sea-Bird Electronics (SBE) 32 Carousel water sampler while sediment samples were obtained with a 300 kg stainless steel day grab sampler. Seawater and sediment samples were aseptically transferred into pre-sterilized containers and stored at 4±2°C for bacterial enumeration and isolation. |
| In the laboratory, water samples were diluted with filtered (0.22 μm) seawater. Sediments were resuspended in filtered seawater to obtain a 1:10 (w/v) dilution, swirled by a vortex, then left to settle; the resulting supernatant was used for microbiological analysis [23]. |
| Total heterotrophic bacteria (THB) were enumerated by spreadplating 0.1ml aliquot of 10-1 to 10-3 dilutions in filtered seawater onto Marine Agar (MA) according to [24]. Similar method was used for enumeration and isolation of hydrocarbon utilizing bacteria (HUB) but the 0.1 ml aliquot was spread-plated on Bushnell-Haas (BH) Agar [25] while the crude oil used as carbon source was introduced by vapour-phase Transfer. Introduction of crude oil by vapour phase transfer was done by placing Whatman No. 1 filter discs impregnated with membrane filter-sterilized crude oil into the lids of Petri dishes. All plating media were incubated in inverted position at 25±2°C for 48 hrs for THB and 7 days for HUB. The bacterial colonies, which developed on the BH agar plates (HUB), were picked and purified by sub-culturing unto fresh MA plates using the streak-plate technique. Axenic cultures were then transferred unto MA slants, properly labeled and maintained as stock cultures. All the isolates were identified based on their cultural morphology, Gram reaction and their biochemical reactions [26-28]. |
| HUB was isolated from oily wastewater reservoir in Imo State, Nigeria and purified on similar agar used for HUB isolates from seawater and sediment samples. This was done to eliminate bias and to obtain HUB isolates with similar growth requirements to those of seawater and sediment HUB isolates. Its degradative capability was compared with those of HUB isolates from deep Atlantic Ocean of the Gulf of Guinea seawater and sediment samples. |
| Four (4) axenic HUB isolates with similar growth, morphological and biochemical characteristics were selected for biodegradation study: HBW12a from seawater surface, HBW12b from seawater bottom, HBS12 from superficial sediment and HMWP from oily wastewater reservoir in Imo State. An inoculum was grown in the marine broth up to an optical density of approximately 1.0 at 560 nm wavelength. |
| Aeration flasks (250 ml) were filled with 99ml Bushneel-Haas Broth basal medium and sterilized by autoclaving at 121°C for 15 minutes. To these flasks (with pH of 7.7) was added 1ml membrane 0.45 μm filter sterilized Bonny light crude oil under aseptic condition [29]. 100 μl of each inoculum was then added to separate flask in triplicates and all flasks were incubated on a Grant OLS 200 orbital/linear shaking bath at 150 revolutions per minute [29] and 25 ± 2°C for a period of 30 days. Uninoculated flasks served as control. |
| Culture flasks were harvested at days 1, 15 and 30 for determination of the quantity as well as individual components of total petroleum hydrocarbon (TPH) degraded. TPH was extracted from each flask with di-chloromethane using separatory funnel [30]. The extracts were analysed by gas chromatography, using Agilent 6890N gas chromatograph (Agilent Technologies) equipped with an FID detector, an Agilent 7683 autosampler and an HP-5 5% Phenyl Methyl Siloxane capillary column (30.0 m x 0.32 mm i.d.) with a nominal film thickness of 0.25 μm. Splitless injection method was used with a deactivated, splitless inlet liner with adsorbent material and taper (Agilent Technologies, P/N 5183-4711). Injection volume was 3 μl and injection temperature 250°C. Helium was used as carrier gas (2 mlmin-1). The column was held at 50°C for 2 minutes. The temperature was then increased at 15°C min-1 to 310°C and held for 15 minutes. This enabled a complete run within 34.33 minutes. The amount of TPH was then determined as a sum parameter of resolved and unresolved components eluted from the GC capillary column between the retention times of n-octane and n-tetracontane. |
| Statistical analysis was performed using Two-way Analysis of Variance (ANOVA) on the data obtained for the quantity and percentage of crude oil degraded. ANOVA was performed among all the treatment at 95.0 confidence levels using MINITAB R14 software. |
| Results and Discussion |
| The THB properties of seawater and sediment samples as well as oily wastewater sample from a reservoir in Imo State are presented in (table 1). Seawater heterotrophs (THB) able to grow on MA were less abundant, with 2.94 x 103 cfu/ml and 1.34 x 103 cfu/ml average counts at the surface and bottom respectively. The resulting average THB values were however higher in sediment (1.03 x 106 cfu/g) and oily wastewater (1.43 x 104 cfu/ml) samples. |
| Microorganisms are essential components of the marine ecosystem and are also involved in the decomposition of accumulated organic matter. Aerobic heterotrophic bacterial (THB) presence in the surface, middle and bottom seawater samples obtained from the Nigeria deep Atlantic Ocean of the Gulf of Guinea has been reported [9]. Several previous studies have also revealed the presence of THB in ocean sediments [14,23,29]. |
| Marine microorganisms play an important ecological role in the process of biological production and cycling of material in marine environments [31]. Less abundance of seawater heterotrophs (THB) observed in this study could be attributed to several factors. Microorganisms are constantly in competition for available nutrients hence their presence in the marine environment is limited by factors such as energy in the form of light and chemical compounds, temperature, nutrients, pressure, pH and salinity. They extract the energy they need through oxidation-reduction reactions. In response to oligotrophic environments (low nutrient level) and intense competition, many micro-organisms become more competitive in nutrient capture and exploitation of available resources [31]. Cavallo et al. [23] stated that the density of heterotrophic bacterial populations in seawater usually ranges from 103 to 106 cfu/l when cultured on artificial medium and concluded that conventional culture methods usually detect only about 10% of the viable THB population. Distance from the nearest Nigerian shore, depth as well as the physico-chemical characteristics of the study site could have contributed to low THB density. |
| The THB population of the seawater column and sediment is in the order sediment>surface> bottom. This order supports the work of [32] who concluded that the bacterial population in the water column decreases with depth and increases at the sea bottom sediment. This was further confirmed by [23] who reported higher bacterial concentration in sediment than in seawater samples. Difference in THB values observed at various depths was significant (p<0.05) but values were consistent across sample stations (p>0.05). Higher THB population recorded in sediment samples can be attributed to the physico-chemical nature of the sediment and the presence of high organic matter concentrations. Generally, microbial populations are more abundant in muddy sediments than in sandy ones depending on the granulometry of particles [23]. |
| HUB density formed up to 1.87% and 1.46% of the THB abundance in seawater surface and seawater bottom respectively while less than 0.1% was observed in sediment. Average HUB count of 7.65 x 103 cfu/ ml which represents 53.5% of THB abundance was however observed in oily wastewater reservoir samples. This is a confirmation of the isolates’ prior exposure to crude oil hydrocarbons. Species of Pseudomonas were clearly dominant HUB in seawater and sediment samples. Similar species type also predominated as HUB in oily wastewater reservoir samples. |
| Although there was significant variation in the HUB density of seawater and superficial sediment samples (p<0.05), the results revealed that these alkane-degrading bacteria (HUB) were present in all deep seawater and superficial sediment samples. Natural sources such as marine seeps and sediment erosion are the main sources of petroleum hydrocarbon in the marine environment. HUB values were generally higher in sediment samples. Presence of alkane-degrading bacteria (HUB) in the deep-seawater column and superficial sediments have been reported by various authors [21,33,34]. However, the percentage of HUB in THB of the seawater and sediment samples (table 1) suggests that the study area was free of petroleum hydrocarbon contamination as at sampling time. |
| Liu and Shao [33] worked on the biodiversity of hydrocarbon degrading bacteria in the deep sea sediment samples of the South Sea of China. Their results suggested that Bacillus was the dominant member in the hexadecane enriched communities and demonstrated that Bacillus aquimaris was the most predominant alkane degrader in all 7 samples at 2 sampling sites. They therefore concluded that species of Bacillus might play an important role in alkane degradation in the deep sea superficial sediments. However, species of Pseudomonas appeared predominant in this study. Morphological and biochemical characteristics of Pseudomonas isolated in this study are similar to those described by various authors [9,24,28,35-37]. Its capacity for rapid growth in the absence of complex growth factors is responsible for its predominance in seawater and sediment [38]. |
| Tapilatu et al. [21] have also isolated alkane-degrading bacteria from the deep sea superficial sediments at the water/sediment interface of about 2,400m water depth. They identified Pseudomonas as one of the predominant heterotrophic bacteria in the deep sea with alkane (TPH) degradative capabilities. |
| Pseudomonas is a well-known and widespread microorganism, which have been isolated from a variety of natural sources and characterized by a high level of metabolic diversity [39-41]. Pseudomonas has also been isolated in the deepest recorded part of the ocean at 11,033m which lies in the Mariana Trench in the Pacific Ocean [42]. Pseudomonas is known to possess physiologic and genetic capabilities for surviving and proliferating under extreme environmental conditions such as alkaline and acidic conditions, high and low temperatures and very high pressure under the deep sea [28]. Possession of multiple genes coding for all the three classes of ribonucleotide reductases in Pseudomonas species is another adaptive feature for their survival both in oxic and anoxic environments [41,43]. Their survival in harsh environments may also be partly due to their hydrophobic nature which is a consequence of the presence of proteinaceous substances in their outermost cell layer [44]. |
| 53.5% of HUB density in entire THB population observed in oily wastewater reservoir samples is an indication of the heterotrophic bacterial isolates’ prior exposure to crude oil hydrocarbons and a confirmation of the chronic presence of crude oil hydrocarbons in the environment. The alkane-degrading bacteria (HUB) from this environment served as basis on which biodegradation capabilities of alkanes (TPH) by HUB from the deep seawater and sediment was assessed. |
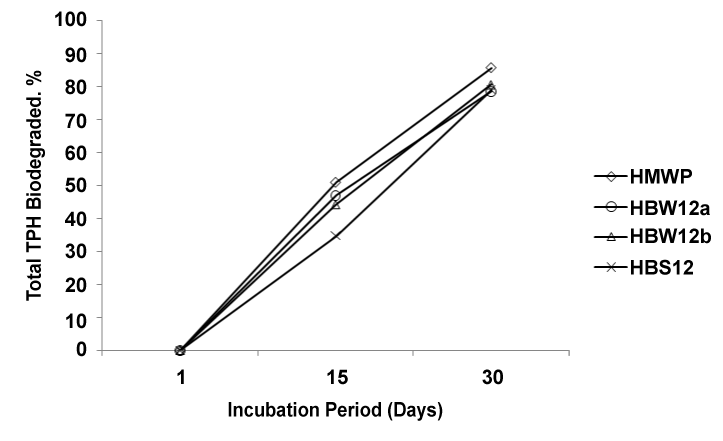
| TPH distribution (Figure 1) was obviously identical in every sample at the beginning of the experiment (day 1). It was demonstrated that TPH fraction of Bonny light crude oil was degraded significantly (P≤0.05) in 30 days by axenic HUB isolates (Pseudomonas sp) from seawater (surface and bottom), sediment and oily wastewater reservoir. |
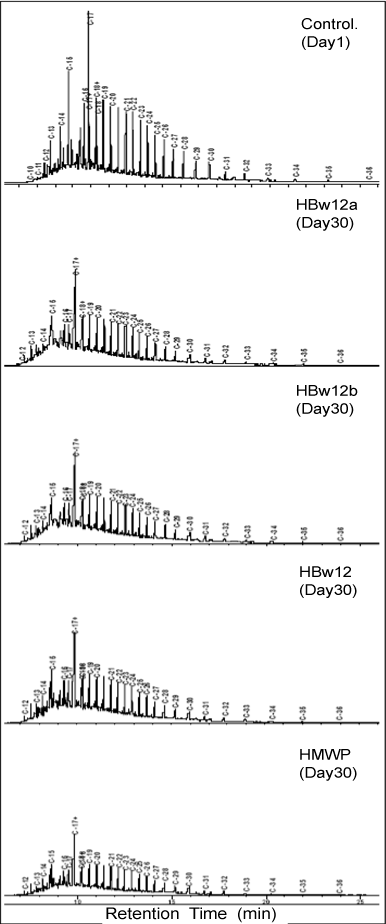
| The order of degradation is HMWP from oily wastewater reservoir > HBW12b from seawater bottom > HBS12 from sediment > HBW12a seawater surface as indicated by valley-valley integration of the resolved peaks in the chromatograms (Figure 2). |
| Although HUB isolate from oily wastewater reservoir showed highest degradative capabilities, similar HUB isolates from seawater surface, seawater bottom and sediment also demonstrated crude oil degradative potential despite their prior non-exposure to crude oil in their natural habitat. Hydrocarbon degradation by natural bacterial flora of a non oil-polluted environment has been reported [29]. Absence of lighter TPH fractions up to n-decane (C10) in all the treatments shows that they might have been evaporated due to their volatile nature [29]. |
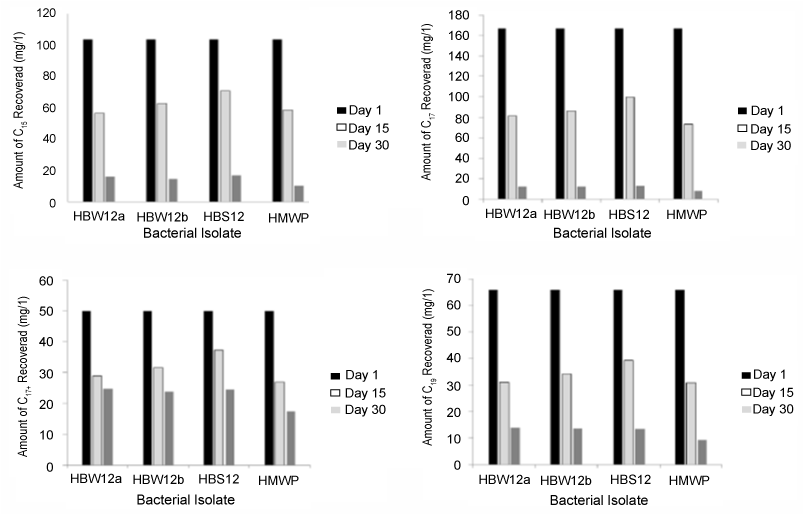
| It appeared though that there was no selective utilization of different fractions of TPH as reduction was observed in all, although some were more reduced than others. A closer look at the degradation of n-C15 (n-pentadecane), n-C17 (n-heptadecane), n-C17+ (n-pristane) and n-C19 (n-nonadecane) fractions of TPH (Figure 3) which showed relatively high concentrations at the onset of the experiment (day 1) revealed that these constituents were all reduced by varying degrees within the study period. This is an indication that the isolates, although morphologically and physiologically similar, possessed broad enzymatic capacity for crude oil hydrocarbon degradation [45]. |
| An estimate of the degree of biodegradation was also observed by comparing GC peak-height ratios of n-C17+ (pristane: a C19 isoprenoid) with n-C17 (n-heptadecane). The n-C17/pristane peak-height ratios of 0.43, 0.47, 0.47 and 0.48 were recorded for HMWP, HBW12a, HBW12b and HBS12 respectively in day 30. |
| The n-C17 /pristane peak-height ratio fell from 3.19 (day 1) to <1.00 (day 30) which indicated that HMWP from oily wastewater reservoir possessed highest degree of TPH degradation. HBW12a and HBW12b from seawater surface and seawater bottom respectively possessed similar degree of TPH degradation which was higher than that of HBS12 from sediment. This is in agreement with [46] who reported that n-alkanes dominate the composition of fresh hydrocarbon while isoprenoids dominate the composition of biodegraded hydrocarbon. All the four isolates tested were able to degrade pristane in 30 days. |
| Conclusion |
| It is evident from this study that bacterial isolates from surface and bottom seawater as well as superficial sediment of the deep Atlantic Ocean of the Gulf of Guinea possessed alkane (TPH) degradative capabilities. The use of indigenous bacteria with petroleum hydrocarbon degradative potential as seed could prove a more environment-friendly bioremediation approach in deep Atlantic Ocean of the Gulf of Guinea. This would on the long run enhance sustainable development rather than the use of exotic bacterial strains and chemicals [22]. |
| In relation to the number of square kilometers of Nigeria segment of the deep Atlantic Ocean and the very few samples which have been taken for this study, our information on the alkane-degrading microorganisms of the deep sea is meager, at best. The collection of more samples from a wider geographical area should yield better knowledge of the diversity and attributes of this bacterial group. Use of modern molecular methods of analysis could as well lead to isolation of new alkane-degrading microorganisms having novel biomolecules with excellent capabilities for biotechnological applications in deep seawater bioremediation. |
| Acknowledgements |
| All samples were obtained during execution of some of the environmental consultancy projects in Fugro Nigeria Limited, Port Harcourt, Nigeria and all analysis were carried out in their Environmental Laboratory in Port Harcourt. We are most grateful to the Managing Director, Dr. G.C. Ofunne for facilitating this work. |
References
|
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 15101
- [From(publication date):
January-2012 - Oct 25, 2025] - Breakdown by view type
- HTML page views : 10444
- PDF downloads : 4657
