Short Communication Open Access
Alcaligenes-The 4T Engine Oil Degrader
| Hardik Pathak* and Kamna Bhatnagar | |
| Department of Biotechnology, Mahatma Gandhi Institute of Applied Sciences, Jaipur, India | |
| Corresponding Author : | Dr. Hardik Pathak Head Department of Biotechnology Mahatma Gandhi Institute of applied sciences, JECRC Campus Tonk Road, Jaipur, India Tel: +919602453825 E-mail: hardikaeshu@gmail.com |
| Received June 24, 2011; Accepted September 12, 2011; Published September 16, 2011 | |
| Citation:Pathak H, Bhatnagar K (2011) Alcaligenes-The 4T Engine Oil Degrader. J Bioremed Biodegrad 2:124.doi: 10.4172/2155-6199.1000124 | |
| Copyright: © 2011 Pathak H et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Microorganisms used in all experiments were isolated by selective enrichment technique. MSM broth was used in enrichment technique supplemented with 1% v/v hydrocarbon substrate (4T engine oil). Bacterial inoculation of biometric flask (containing 48 ml minimal salt medium) consisted of 1ml of a 48 hours culture. Hydrocarbon substrate (4T engine oil) was then added to 1% v/v. The GC/MS analysis was performed using a MS 5973 spectrometer coupled to 9 Hewlett Packard model 6890. I4 isolate microorganisms degraded around 76% of 4T engine oil. Gas chromatography showed significant differences in the composition of hydrocarbons at the end of the experiments.
| Keywords |
| Alcaligenes; GC-MS; Spectrophotometer; MSM |
| Introduction |
| During the previous years, the frequency and risk of oil pollution has led to extensive research. Most of the petroleum goes in the ecosystem via leakage of coastal oil refineries. This fact evoked the interest of scientists to investigate the oil distribution system and its fate in the environment, especially the marine environment. Approximately five million tons of crude oil and refined oil enter the environment each year as a result of anthropogenic sources such as oil spills [9]. Past analysis of reported oil spills indicate that most of the oil comes from tankers, barges and other vessels as well from land pipeline spills. Extensive changes in marine, as well as terrestrial ecosystems resulting from the grounding of the Exxon Valdez. The Nahodka oil spill, the Erica spill and the Prestige spill, have recently increased the attention of environmentalists, chemists, biotechnologists and engineers [3,23]. |
| PAHs present as natural constituents in fossils fuels, are formed during the incomplete combustion of organic material and are therefore present in relatively high concentration in products of fossil fuels refining [1,4,13,17,18,26,27]. Petroleum refining and transport activities are major contributors to localized loadings to PAHs into the environment. Such loadings may occur through discharge of industrial effluents and through accidental release of raw and refined products. However, PAH released into the environment may originate from many sources including gasoline and diesel fuel combustion [14,15] and tobacco smoke [6]. PAHs are detected in air [11,14], soil and sediment [8,12,19,20,24,28], surface water groundwater, and road runoff [2,10,16,21] are dispersed from the atmosphere to vegetation [25] and contaminate foods [5,13,22]. Anthropogenic and natural sources of PAHs in combination with global transport phenomena result in their world wide distribution. Hence, the need to develop practical bioremediation strategies for heavily impacted sites is evident [7]. |
| Bioremediation is defined as use of biological processes to degrade, breakdown, transform, and/or essentially remove contaminants or impairments of quality from soil and water. Bioremediation is a natural process which relies on bacteria, fungi, and plants to alter contaminants as these organisms carry out their normal life functions. Metabolic processes of these organisms are capable of using chemical contaminants as an energy source, rendering the contaminants harmless or less toxic products in most cases. This investigation focuses on the general processes of bioremediation with in the soil environment and highlighting the biodegradation of petroleum hydrocarbons. |
| Materials and Methods |
| 4T engine oil (mainly composed of medium chain length of hydrocarbon) obtained from Bharat petrol pump. The entire chemical was purchased from MERCK. MSM (Minimal Salt Media) media containing 1 g K2HPO4, 1g NH4NO3, 1 g KH2PO4, 0.02 g CaCl2, 0.05 g FeCl3, 0.2 g MgSO4 was used for serial dilutions to determine viable cell counts. For isolation and enumeration of total viable cells MSM agar plates were used. Microorganisms used in all experiments were isolated by selective enrichment technique. MSM broth was used in enrichment technique supplemented with 1% v/v hydrocarbon substrate (4T engine oil). The hydrocarbon substrate used in enrichment methods was a mixture of hydrocarbons. After one week of incubation on rotary shaker at 27ºC and 200rpm, 10ml of sample from primary enrichment was transferred to a fresh MSM broth. Unless otherwise stated, after 2nd enrichment 1ml of medium was plated after appropriate dilution on MSM agar plate and incubated at 27ºC. After 48 hour incubation, pure colonies were isolated by using single colony isolation method. Isolated colonies were stored at 4ºC and re-plated at MSM agar plates at 3 weeks interval. Bacterial isolates not used in biodegradable experiments were mixed with 40% glycerol and stored at 70ºC for future use. The initial number of total viable cells in the original sample (before enrichment) was determined by serial dilution agar plating procedure Bacterial inoculation of biometric flask (containing 48ml minimal salt medium) consisted of 1ml of a 48 hours culture. Hydrocarbon substrate (4T engine oil) was then added to 1% v/v. The sidearm of biometric flask was fitted with 10 ml of 0.1 M KOH flask and was incubated at 27ºC, non-shaking. |
| Sample for measuring carbon dioxide were taken by syringe in scheduled time periods from a side arm of the flask. Evolution of carbon dioxide during microbial utilization of 4T engine oil and organic waste was determined by colorimetric titration. The amount of trapped CO2 in KOH was accurately titrated after addition of 1ml of saturated barium chloride and 0.1ml of phenolphthalein with 0.05M HCL until colorless solution. The control sample contained 10ml of fresh KOH with 1ml of barium chloride and 1ml of phenolphthalein. The volume of HCL needed for neutralization of the experimental KOH was subtracted from the amount of HCL needed for neutralization of the un-exposed KOH. The difference in milliliters was converted into micromoles of evolved carbon dioxide during microbial degradation of hydrocarbons. |
| Sets of test tubes experiments were designed in order to quantitatively analyze the microbial hydrocarbon degradation at GC-MS. Tube containing cultures of bacteria and hydrocarbon were incubated during the same time period and in the same experimental conditions as biometric flask. Each test tube contained 4ml of sterilized minimal salt medium, 80μl of microbial inoculums and 50μl of hydrocarbon substrate. The sample extraction was carried out by mixing the entire volume of one set tube (approximately 5ml) with 1 ml of hexane (petroleum fraction). This mixture was emulsified by shaking and allowed to resettle for five minutes. The top layer was recovered and transformed to a clear vial for further use. Mass spectra as well the retention times of standard mixture of hydrocarbons were used to quantify each analyte (table 1). |
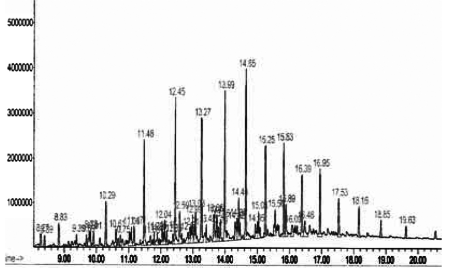
| The GC/MS analysis were performed using a MS 5973 spectrometer coupled to 9 Hewlett Packard model 6890, with a column ULBON HR-1 which is equivalent to Ov-1 fused silica capillary (.25mm x 50mm) with thickness of 0.25 micron; 1ml/min; pressure 18.5 psi and split ratio 20%. The initial temperature was 70ºC kept for 5 minutes with a temperature range of 14ºC per minute and final temperature for 280ºC kept for 10minutes with total running time 30 minutes. The solvent used in analysis was hexane (petroleum fraction) (Figure 1). |
| Soil samples were collected from coastal area of Mumbai in the month of July, 2005. Samples were collected from Sai Motor Garage nearby Mumbai High; as such samples were highly polluted with engine oil. |
| Results and Discussion |
| Prior to the screening of hydrocarbon degrading microorganisms, the microbial populations were estimated in each original sample. Appreciable numbers of bacteria up to 1010 colony forming units (CFU) have been found. Indigenous organisms isolated in this study were selected by enrichment culturing technique. As the results in indicates a significant increase in hydrocarbon degrading microorganisms in PCS–1, after the first and second week of enrichment in 4T engine oil. In PCS I original sample the CFU was 1.3×1010 before adding the substrate. As the substrate 4T engine oil was added to the sample, the CFU was 8.3×1010 after 1st enrichment, and again increased when 2nd enrichment was performed (9.4×1010). In second sample PCS II, the CFU was 1.6×109 before enrichment culture. After 2nd and 3rd enrichment it increased up to 6.4×109 and 7.3×109 respectively. Due to high diversity of microorganisms after second enrichment 15 bacterial isolates were collected from the samples. All these isolate were tested for their ability to utilize various hydrocarbons. Five bacterial strains were found to be the best degrader of 4T engine oil. |
| The type of enrichment substrate significantly affected the microbial population. It is observer that the large and more complex the structure of hydrocarbon, the more slowly is oxidized. This may depends on the type of organisms involved and the medium, in which it was developed. For this reason, the longer enrichment period as well as fresh medium and decantation of toxic co-metabolites apparently enhanced the proliferation of bacteria capable of utilization more complex compounds in investigated samples collected from coastal area Mumbai. |
| The morphology and type of bacterial colonies were also investigated in cell counts experiments. One of the objectives of this study was to isolate as many culturable strains as possible in standardized culture condition. For this reason, a first screening of strain was done after gram staining and microscopic examination for bacteria to eliminate apparently similar strains. As the results exhibited original sample were characterized with a very high diversity of microorganisms. The metabolic diversity of microorganisms in the natural environments is an important factor in the biodegradation of hydrocarbon. Extensive degradation of petroleum pollutants is generally accomplished by mixed microbial population, rather than single microbial species (Table 2). |
| Even though enrichment culturing selected only those indigenous microorganisms that have been especially acclimated to degrade hydrocarbons, it was necessary to characterize the biodegradation potential for individual isolates. For this reason, each isolated microbial strain was submitted to a preliminary batch flask test experiment for detailed investigation of hydrocarbon utilization. The structure of a compound is important in determining its biodegradability. Generally, straight chain alkanes are degradable more readily than branched alkanes. In order to investigate the capabilities of isolates to utilize both chemical structures, liner as well as branched, 4T engine oil was used as a substrate. The growth was monitored regularly by measuring optical density of experimental medium. Among all the isolated microbial strains, biodegradation potential was recorded ranging from 44% to 91% degradation of 4T engine oil. Considering the fact that some of these microorganisms have been not exposed to hydrocarbons at all or only in very low concentration, it is very possible that the adaptation process might play an important role in degradation process. There is high degree of variability in the ability of a microbial community to adapt to compounds and chemicals that they were never exposed to before. Adaptation depends on many factors, such as the induction or de-repression of enzymes specific for the degradation pathway of a particular compound or an adaptation of existing catabolic enzymes to the degradation of novel compounds (Table 3). |
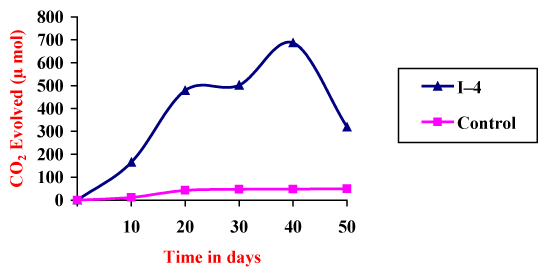
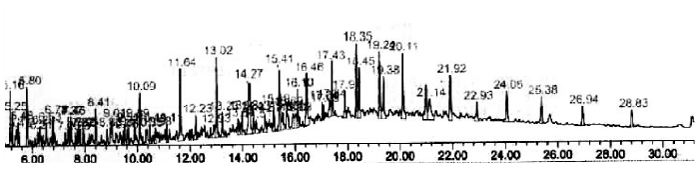
| Effective method to study the degradation of hydrocarbon by microorganisms is to measure the amount of carbon dioxide evolve during utilization of organic compound. Respiratory activities of isolated microorganisms were measured during growth on addition of 4T engine oil in a biometric flask. The cumulative amounts of CO2evolved during mineralization of 4T engine oil were measured. The I4 isolate exhibited lower production rates during first 12days. Maximum CO2 evolution (867μmol) reached after 30 days, followed by a very rapid decline within next 10days. I4 isolate microorganisms degraded around 76% of 4T engine oil (Figure 2). Gas chromatography showed significant differences in the composition of hydrocarbons at the end of the experiments (Figure 3) (Table 4). |
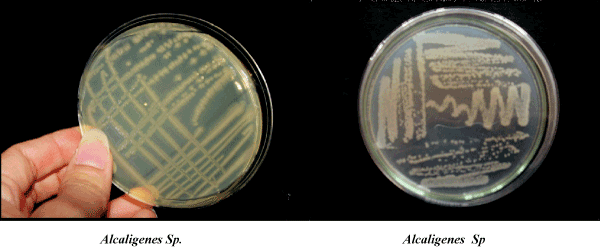
| Among the identified strains identified, I4 strain was rods, cocci in structure. It had irregular margin, viscous growth, flat elevation and light cream in colour. It showed positive response for Sucrose utilization, Catalase, Nitrate reduction, Oxidase, Gelatin hydrolysis, Starch hydrolysis, Citrate utilization. It was negative for Urease activity, Indole, Voges-Proskauer, Sulphate production, Methyl red, and Lactose utilization. This strain was identified as Alcaligenes Sp (Table 5). |
| Acknowledgements |
| We wish to thank Prof. D. P. Jaroli, Department of Zoology and Prof. C. K. Ojha, Director Academics, M.G.I.A.S., for his encouragement and support. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 | Table 5 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 15610
- [From(publication date):
September-2011 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 10950
- PDF downloads : 4660
