Aging: The Mitochondrial Connection
Received: 17-May-2012 / Accepted Date: 18-May-2012 / Published Date: 21-May-2012 DOI: 10.4172/2161-0681.S4-003
317438Introduction
Aging is broadly defined as a decline in the functional capacity of both individual cells and entire tissues over time. This decline in function leads not only to an increase in disease risk, but also to the eventual demise of the organism. Although the aging process is still not fully understood, events at the subcellular level are increasingly implicated in promoting or allowing the progressive deterioration during aging. The mitochondrion, an organelle typically presented as the “powerhouse of the cell,” has received a great deal of attention as a driving cause of aging in cells and tissues. With increased understanding of how mitochondria decline with age, there are increased opportunities for therapeutic interventions that could not only improve mitochondrial function, but benefit the entire cell, tissue, and organism. Here, we review different aspects of mitochondrial changes with aging (Figure 1), as well as attempts to limit or reverse mitochondrial dysfunction to prevent aging (Figure 2).
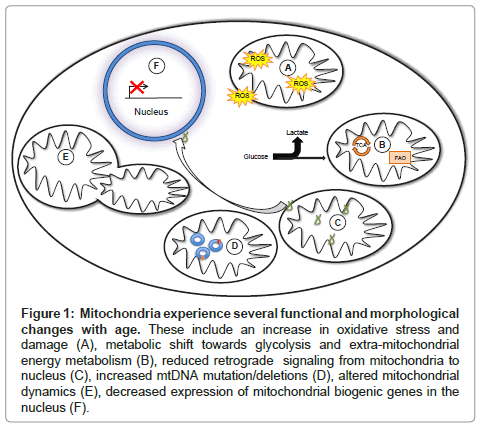
Figure 1: Mitochondria experience several functional and morphological changes with age. These include an increase in oxidative stress and damage (A), metabolic shift towards glycolysis and extra-mitochondrial energy metabolism (B), reduced retrograde signaling from mitochondria to nucleus (C), increased mtDNA mutation/deletions (D), altered mitochondrial dynamics (E), decreased expression of mitochondrial biogenic genes in the nucleus (F).
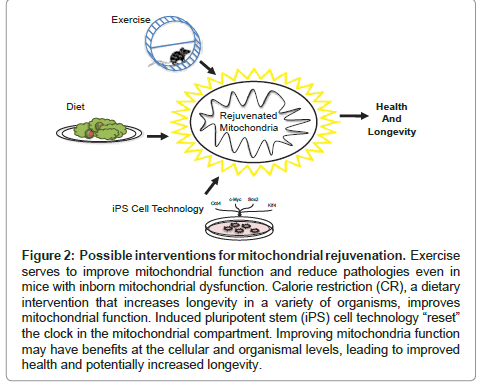
Figure 2: Possible interventions for mitochondrial rejuvenation. Exercise serves to improve mitochondrial function and reduce pathologies even in mice with inborn mitochondrial dysfunction. Calorie restriction (CR), a dietary intervention that increases longevity in a variety of organisms, improves mitochondrial function. Induced pluripotent stem (iPS) cell technology “reset” the clock in the mitochondrial compartment. Improving mitochondria function may have benefits at the cellular and organismal levels, leading to improved health and potentially increased longevity.
Changes in Mitochondria with Aging ROS
A prominent theory proposes that sometime in the early evolution of eukaryotes, an endosymbiotic event led to the formation of mitochondria [1,2]. This event allowed eukaryotic cells to perform oxidative respiration, conferring an evolutionary advantage as evidenced by the explosion of eukaryotic cell life forms. However, mitochondria also became the major site of reactive oxygen species (ROS) production that could, if uncontrolled, level considerable damage on the mitochondria and potentially the rest of the cell [3]. Extrapolated to a multi-cellular organism, ROS could be detrimental to tissue integrity and function, especially if oxidative damage were allowed to accumulate over time. In order to combat this problem, the mitochondrion has a well-developed cadre of antioxidant enzymes and molecules to defend against ROS production. For example, it has its own isoforms of superoxide dismutase (SOD2 or MnSOD), and glutathione peroxidase [4,5].
The ability of mitochondria to defend against oxidative stress is not necessarily maintained throughout aging. Endogenous antioxidant systems, especially in the mitochondria, are often found to be downregulated with age [6,7]. Aged tissues often have high levels of oxidative damage, and oxidative stress has been implicated in the progression of age-related diseases such as diabetes, heart disease, hearing loss, cancer, and various neurological disorders [7-11]. These observations support the “Mitochondrial Free Radical Theory of Aging”, which proposes that oxidative stress and increasing mitochondrial dysfunction create a vicious cycle that promotes the deterioration of the cell and eventually, the deterioration of tissues and the whole organism [12,13].
Despite the elegant simplicity behind the “Mitochondrial Free Radical Theory of Aging”, it is not without controversy. Numerous studies have shown that the relationship between oxidative stress, aging, and longevity is tenuous at best. A comparison of rats and pigeons, similarly sized endotherms that have a seven-fold difference in longevity, showed no overt difference in ROS production and antioxidant levels in various tissues [14]. However, rats, which are shorter-lived, show increased oxidative damage to fatty acids. The naked mole rat (Heterocephalus glaber), a curious rodent with a lifespan of up to 30 years or more, shows intriguing patterns in oxidative stress and damage. Compared to closely related species with much shorter lifespans, such as mice and rats, the naked mole rats produce comparable amounts of oxygen radicals and, surprisingly, exhibits higher biomarkers of oxidative damage [15,16]. However, the naked mole rats differ in their susceptibility to oxidative stress: cells from the naked mole rats have increased resistance to oxidative stress-induced apoptosis [17]. Thus, a confounding factor to oxidative stress is the capacity of cells to resist oxidative stress.
If oxidative stress and damage are indeed causal to aging, reducing oxidative stress and damage should slow or reverse aging. However, several studies indicate just the opposite. Exogenous antioxidant treatments are not helpful in promoting longevity or preventing age-related diseases [18-22]. Strikingly, overexpression of several major antioxidants, including the various isoforms of SODs, as well as catalase, an antioxidant enzyme in the peroxisome, did not lead to increased lifespan [23].
Some explanations as to why antioxidant treatments remain ineffectual include technical considerations, such as permeability through membranes and bioavailability of the antioxidant to the sites of oxidative stress [24]. In support of this idea, the targeted expression of catalase to the mitochondria clearly offers protection against mitochondrial oxidative stress and age-related decline in mitochondrial function in mice [25]. Similarly, the antioxidant compound SkQ1, whose positive charge allows targeting to the negatively charged intermembrane space of the mitochondria, is successful in reducing mitochondrial oxidative stress and decelerating senescence [26,27]. Additionally, overexpression of the cytosolic antioxidant thioredoxin 1 in mice conferred clear protection against oxidative damage, as well as increased survivability at earlier life stages [28].
Another possibility is that merely increasing the levels of the antioxidative enzymes is not sufficient to effectively increase antioxidative capacity. The antioxidative enzymes may need to be modified to function at full potential. For example, overexpression of SOD2 only modestly reduces cellular ROS levels. However, deacetylated SOD2 has dramatically increased capacity to dampen cellular ROS [29]. Thus, despite some evidence inconsistent with the Free Radical Theory of Aging, oxidative stress is still likely to be a major cause of aging.
mtDNA Damage
Mitochondria are unique among the organelles in that they sustain their own genomes. The mitochondrial genome is limited to the expression of rRNAs, tRNAs, and genes coding for mitochondrial proteins, most of which are subunits in the complexes of the electron transport chain (ETC) (as reviewed in [30]). Damage or mutations to the mtDNA can accumulate clonally, leading to respiratory chain deficiencies in tissues [31-33]. mtDNA damage is widely believed to be created by ROS: the proximity of the mtDNA to the ROS-producing ETC induces damage and mutations in the mtDNA. These mtDNA mutations, if accumulated to a sufficient level, can lead to inefficient ETC and increased ROS production. This process becomes a vicious cycle [34].
Much has been learned about mtDNA mutation and aging from the mtDNA mutator mice, which express an error-prone version of the catalytic subunit of mtDNA polymerase. These mice show accumulation of mtDNA mutations and accelerated aging phenotypes [35]. Strikingly, in non-dividing tissues, the amount of ROS produced is normal and there is no increased accumulation of oxidative damage [36]. However, the mtDNA mutator mice contain dysfunctional somatic stem cells, which can be rescued by antioxidant treatment [37]. Thus, mtDNA mutations cause aging by increasing cellular ROS levels in somatic stem cells, which are particularly sensitive to oxidative stress and crucial for the aging process. In addition to stem cell defects, mtDNA mutator mice also have deficiencies in several ETC complexes [38], which indicates that dysregulated energy production and metabolism may also contribute to their premature aging phenotypes.
Energy Production and Metabolism
Mitochondria are often affectionately nicknamed “ the powerhouse of the cell.” The epithet is well earned, as one of the most important contributions of the mitochondria to the cell is the production of ATP through oxidative phosphorylation. It has been observed that aged mitochondria have diminished ATP production [39]. The expression of mitochondrial genes is usually downregulated in aged rats and mice, which may account for diminished mitochondrial ATP production [40,41]. Interestingly, the long-lived naked mole rats do not have a reduction in mitochondrial gene expression with age.
The diminished ATP production by aged mitochondria poses a problem for the cell: aged cells must either be able to survive on less ATP or they must shift their metabolism toward extra-mitochondrial energy production. Recent work by Houtkooper et al. indicates that the latter may be occurring [42]. Among their findings was evidence that aged mouse liver and muscle have reduced glycolytic intermediates, but more lactate, indicating a higher reliance on anaerobic metabolism through glycolysis. This reliance on anaerobic glycolysis is in keeping with their observations that several genes that code for mitochondrial proteins are transcriptionally downregulated with age, including genes involved in fatty acid import and oxidation, as well as genes in the ETC. These findings are consistent with work done on dog liver tissue, where glycolytic genes were found to be upregulated, but ATPase was downregulated [43]. This increased dependence on glycolysis with age may be a conserved phenomenon in humans, as suggested by work on aged skin keratinocytes [44].
Consistent with changes in metabolism, the cellular redox status changes with age. In several tissues of aged wistar rats, both the total cellular NAD+ pool and the NAD+:NADH ratio were diminished compared to young rats [45]. These changes in redox status can have dramatic effects on aging. For example, this environment is prohibitive for the activity of NAD+-dependent enzymes, such as the sirtuins, which extend lifespan in model organisms. It would be interesting to know if the mitochondrial NAD+ and NADH pools are altered with age.
Structure and Dynamics
Mitochondria have very distinct structures, consisting of a double membrane construction and a highly folded internal membrane forming the “cristae”. Mitochondria form adaptable networks, which fuse and break apart in turn. These mitochondrial dynamics have been increasingly appreciated for their role in healthy mitochondrial maintenance. Deficient fusion or fission causes severe mitochondrial dysfunction, which has profound physiological relevance, such as neurodegeneration and muscle atrophy [46-50]. Aged tissues were found to harbor giant mitochondria [51-53], suggesting that mitochondrial dynamics may be altered in aged tissues, contributing to mitochondrial decline with age.
Maintaining structure within the mitochondria also has functional consequences. The major site for oxidative phosphorylation (OXPHOS) is the cristal membrane. The organization of the cristae into welldefined cristal junctions creates a thermodynamically favorable environment for efficient ETC and ATP production [54]. Whereas the muscle mitochondria of young rats have well delineated cristal structures, the muscle mitochondria of aged rats display undefined cristae [55]. These structural defects may result in an age-dependent decline in mitochondrial function.
Mitochondria not only interact with each other, but can physically interface with other organelles, such as the endoplasmic reticulum (ER) [56]. This interface can affect calcium pools and calcium-related signaling within these organelles. The ER-mitochondria foci also allow for direct exchange of phospholipids necessary to maintain the mitochondrial inner and outer membranes. These interactions may represent a potentially important mitochondrial maintenance mechanism during aging. Indeed, an alternate explanation for the agerelated swollen “giant mitochondria” is increased calcium signaling and aberrant opening of the mitochondrial permeability transition pores [57].
Mitochondrial-nuclear Crosstalk
Although mitochondria have their own DNA, the coding regions are only responsible for a small fraction of the total mitochondrial proteins; nuclear genomic DNA code for the remainder. Therefore, it is necessary for the nucleus and mitochondria to communicate their adaptive needs as the cell experiences different stressors. “Mitohormesis” is a concept that arose to describe the retrograde cellular response to mild stresses in the mitochondria. A mild insult occurring in the mitochondria, such as low levels of oxidative stress, results in the cell mounting a protective response to manage the insult. The net outcome is actually beneficial to the cell. This process is very much dependent upon mitochondrial-nuclear communication and is thought to underlie the life-extension benefits of low glucose dietary interventions [58,59].
The rhomboid protease PARL, found in the inner mitochondrial matrix, has the ability to cleave itself, creating a PARLb peptide, which is targeted to the nucleus to promote the transcription of mitochondrialrelated nuclear factors (PGC1-b, NRF1, and MFN1) and increase mitochondrial mass [60]. PARL appears to be relevant to mitochondrial aging as PARL expression was found to be downregulated in aged human skeletal muscle biopsies [61]. The decreased expression of PARL with age may lead to a decreased ability to communicate mitochondrial bioenergetic signals back to the nucleus through the PARLb peptide.
Another mitochondrial-nuclear crosstalk pathway was uncovered in Retinal Pigment Epithelial (RPE) cells. Under oxidative stress conditions, the mitochondrial protein prohibitin is shuttled from the mitochondria to the nucleus, creating anti-apoptotic conditions to protect the cells from premature cell death [62]. Interestingly, prohibitin expression is decreased in RPE cells with age, indicating that this defense mechanism is suppressed with age.
The Promise of Rejuvenation
The possible causal role of declining mitochondrial function to aging raises the question of whether rejuvenation can be achieved by improving mitochondrial function. This section of the review will focus on methods to improve mitochondrial function and speculate on whether any of these methods could potentially be used to rejuvenate cells and extend lifespan (Figure 2).
CR, SIRT3, and Mitochondria
It has been known for decades that Calorie Restriction (CR), a diet that consists of reduced caloric intake without malnourishment, extends lifespan in rodents, and recent work has shown that CR extends lifespan in yeast, worms, and flies among other organisms [63]. CR has even been shown to dramatically improve health and cognition and reduce age-related mortality in primates [64]. While the mechanisms behind how CR improves health and extends lifespan are not completely known, studies have consistently shown that CR leads to a reduction in mitochondrial oxidative stress [65]. Originally, the reduced oxidative stress in mitochondria was thought to be due to a decreased metabolic rate, resulting in a lower production of ROS, and some evidence supports this theory [66,67]. However, other studies indicate that mitochondrial activity actually increases in organisms undergoing CR [68,69]. Indeed, CR promotes mitochondrial biogenesis via eNOS [68]. Thus, CR increases the amount of mitochondria and can lead to an increase in their metabolic rate. How then do mitochondria reduce oxidative stress under CR?
Clues to the resolution of this puzzle came from reports that during CR, the ability of mitochondria to scavenge ROS improves, and this ability depends on the activity of the sirtuin SIRT3 [10,29]. The sirtuins are an evolutionarily conserved family of NAD+-depended deacetylases that have been shown to promote lifespan in model organisms when overexpressed [70]. SIRT3 is one of seven mammalian sirtuins and one of three that are localized to the mitochondria [71]. CR increases the expression and activity of SIRT3 [29,72]. SIRT3 deacetylates superoxide dismutase 2 (SOD2) and isocitrate dehydrogenase 2 (IDH2), increasing their activities, which are essential for reduction of oxidative stress in the mitochondria. In the absence of SIRT3, the reduction in oxidative stress during CR is abrogated [10,29].
From a physiological standpoint, SIRT3 has been shown to be crucial for the ability of CR to stave off age-related hearing loss [10]. Hearing loss is a degenerative condition that occurs with aging and is at least partially driven by oxidative damage arising from mitochondria. Mice fed a CR diet show a delay in the deterioration of hearing that is abrogated in the absence of SIRT3 [10]. The study directly implicated oxidative stress from mitochondria in a pathology of aging and showed that this condition can be ameliorated by a dietary regimen.
The importance of SIRT3 for mitochondrial health is also demonstrated by studies in the heart and the kidneys. Mice deficient in SIRT3 show premature aging in the heart, due to increased oxidative stress and dysregulation of the mitochondrial permeability transition pore. This results in hypertrophy and fibrosis in the heart well before the age at which they normally occur in wild type hearts [73,74]. Oxidative stress from the mitochondria also plays a detrimental role in kidney function as evidenced by mice fed a high fat diet, which exhibit strong kidney inflammation. This inflammation was abrogated by enforced expression of SIRT3, which improved mitochondrial function and the health of the kidney cells [75].
The benefits of CR are at least partially mediated by improved function of mitochondria. SIRT3 is crucial for maintaining mitochondrial health and for reducing oxidative stress in mitochondria under CR. Thus, SIRT3 serves as an intriguing target for mitochondrial rejuvenation. While CR poses practical limitations in human implementation due to its effect on the quality of life, pharmaceutical activators of proteins activated by CR, such as SIRT3, may provide most of the beneficial effects of CR with none of the hunger pains. A study of mitochondrial health and lifespan in SIRT3 transgenic mice will be informative with regards to assessing the feasibility of this approach.
Rejuvenation through Exercise
The idea of rejuvenation through exercise has been tested with the mitochondrial mutator mice [35]. When the mice were placed on an endurance exercise regimen for 5 months, they showed substantial improvements in mitochondrial function along with much improved physiology [76]. Although the molecular mechanisms underlying these drastic systemic changes are not well understood, it is thought that endurance exercise may increase the activity of PGC-1α, which has been speculated to increase mitochondrial biogenesis and improve clearance of damaged mitochondria [76].
Next-generation Interventions
More advanced molecular interventions hold even more promise for rejuvenation. A cocktail of 4 transcription factors has been shown to restore the differentiation capacity of cells, including cells from very aged donors, into cells that have attributes of embryonic stem cells (ESCs), termed induced pluripotent stem (iPS) cells [77]. Recent work indicates that the process of inducing pluripotency also rejuvenates the energetic capacity of the aged cells, and dramatically improves their mitochondrial function [78,79]. Mitochondria from the iPSC cells of centenarian donors are functionally and morphologically indistinguishable from the mitochondria of Embryonic Stem Cells (ESCs). Thus, the decline in mitochondrial function with age is fully reversible.
Spatial and Temporal Mitochondrial Regulation of Aging
While it is clear that increasing mitochondria dysfunction contributes to the decline of tissue integrity and the progression of aging, it is unclear whether mitochondrial health is equally important across all tissue and cell types. Tissue-specific stem cells are responsible for the maintenance and repair of the tissues of an organism throughout its lifespan. Given this monumental task, it is probable that these cell populations are more sensitive to age-related mitochondrial dysfunction. Aged stem cells have fewer mitochondria, reduced oxidative metabolism, and increased oxidative stress [80]. Numerous mouse models with defective management of ROS have compromised stem cell functions, which can be rescued by antioxidant treatments [81-83], indicating the critical importance of mitochondrial oxidative stress in stem cell and tissue maintenance during the aging process. As discussed above, stem cells are particularly sensitive to mitochondrial damage and oxidative stress compared to post-mitotic tissues.
Mitochondria in different tissues may differentially contribute to organismal longevity. In D. melanogaster, overexpression of PGC-1 in the digestive tract has been shown to improve mitochondrial function and increase longevity [84]. Yet, there is no increased longevity for overexpression in neurons, muscle, or upon ubiquitous overexpression of PGC-1.
Elegant studies in C. elegans provide high resolution spatial and temporal views of mitochondrial regulation during the aging process [85]. Although compromised mitochondrial function is thought to contribute to aging, lifespan can also be extended by reducing mitochondrial function, and in particular, the function of ETC components. However, reduced ETC modulates the aging process in tissue-specific and temporal-specific manners. The L3/L4 larval developmental period, when mitochondria undergo dramatic proliferation, is a critical period in which reduced ETC modulates the aging process. Suppressing the ETC components before but not after this developmental stage induces the mitochondrial stress response and extends lifespan. Not all tissues are equally responsive to the ETC-induced lifespan extension. For example, reducing the ETC in neuronal and intestinal tissues but not muscle extends lifespan. Strikingly, mitochondrial stress in one tissue can produce a signal that is transmitted to a distal tissue to induce mitochondrial stress. Thus, mitochondrial perturbation can modulate the aging process in a non cell-autonomous fashion.
Conclusion and Future Perspectives
The mysteries shrouding the aging process are slowly being unraveled by the powerful tools of molecular and cell biology. As we have learned more about the mechanisms that contribute to aging at the molecular level, we have begun to appreciate the complexity of aging and have come to familiarize ourselves with the key players in the process. Mitochondria are surely among the most important of these players. However, the role of mitochondria in aging is complex, as highlighted by observations that reducing oxidative stress may not be sufficient to extend lifespan. Indeed, extension of lifespan may require improved mitochondrial function, which paradoxically, can be induced by mildly stressing the mitochondria, a concept known as mitohormesis.
The question then is whether there is an optimal level of oxidative stress in mitochondria to induce peak function. And, if that optimal level of stress exists, which tissues would benefit most from improved mitochondrial function and at what timeframe? At a smaller scale, what cell types in the tissues would make the best targets? Somatic stem cells, which are responsible for maintaining tissues throughout the life of an organism, would appear to be prime targets for interventions to improve mitochondrial function. Much work remains to be done to answer these questions, which will form the basis for developing mitochondrial interventions to improve health and extend lifespan.
References
- Sagan L (1967) On the origin of mitosing cells. J Theor Biol 14: 255-274.
- Bhattacharya D, Archibald JM, Weber AP, Reyes-Prieto A (2007) How do endosymbionts become organelles? Understanding early events in plastid evolution. Bioessays 29: 1239-1246.
- Whiteman M, Dogra Y, Winyard PG, Armstrong JS (2009) Detection and measurement of reactive oxygen intermediates in mitochondria and cells. Methods Mol Biol 476: 28-49.
- Melov S (2000) Mitochondrial oxidative stress. Physiologic consequences and potential for a role in aging. Ann N Y Acad Sci 908: 219-225.
- Panfili E, Sandri G, Ernster L (1991) Distribution of glutathione peroxidases and glutathione reductase in rat brain mitochondria. FEBS Lett 290: 35-37.
- Bai XY, Ma Y, Ding R, Fu B, Shi S, et al. (2011) miR-335 and miR-34a Promote renal senescence by suppressing mitochondrial antioxidative enzymes. J Am Soc Nephrol 22: 1252-1261.
- Castro Mdel R, Suarez E, Kraiselburd E, Isidro A, Paz J, et al. (2012) Aging increases mitochondrial DNA damage and oxidative stress in liver of rhesus monkeys. Exp Gerontol 47: 29-37.
- Zhang L, Ebenezer PJ, Dasuri K, Fernandez-Kim SO, Francis J, et al. (2011) Aging is associated with hypoxia and oxidative stress in adipose tissue: implications for adipose function. Am J Physiol Endocrinol Metab 301: E599-607.
- Cocheme HM, Quin C, McQuaker SJ, Cabreiro F, Logan A, et al. (2011) Measurement of H2O2 within living drosophila during aging using a ratiometric mass spectrometry probe targeted to the mitochondrial matrix. Cell Metab 13: 340-350.
- Someya S, Yu W, Hallows WC, Xu J, Vann JM, et al. (2010) Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 143: 802-812.
- Finley LW, Carracedo A, Lee J, Souza A, Egia A, et al. (2011) SIRT3 opposes reprogramming of cancer cell metabolism through HIF1a destabilization. Cancer Cell 19: 416-428.
- HARMAN D (1956) Aging: a theory based on free radical and radiation chemistry. J Gerontol 11: 298-300.
- Harman D (1972) The biologic clock: the mitochondria? J Am Geriatr Soc 20: 145-147.
- Montgomery MK, Hulbert AJ, Buttemer WA (2011) The long life of birds: the rat-pigeon comparison revisited. PLoS One 6: e24138.
- Pérez VI, Buffenstein R, Masamsetti V, Leonard S, Salmon AB, et al. (2009) Protein stability and resistance to oxidative stress are determinants of longevity in the longest-living rodent, the naked mole-rat. Proc Natl Acad Sci U S A 106: 3059-3064.
- Andziak B, O'Connor TP, Qi W, DeWaal EM, Pierce A, et al. (2006) High oxidative damage levels in the longest-living rodent, the naked mole-rat. Aging Cell 5: 463-471.
- Labinskyy N, Csiszar A, Orosz Z, Smith K, Rivera A, et al. (2006) Comparison of endothelial function, O2-* and H2O2 production, and vascular oxidative stress resistance between the longest-living rodent, the naked mole rat, and mice. Am J Physiol Heart Circ Physiol 291: H2698-2704.
- Bernhard D, Wang XL (2007) Smoking, oxidative stress and cardiovascular diseases--do anti-oxidative therapies fail? Curr Med Chem 14: 1703-1712.
- Magwere T, West M, Riyahi K, Murphy MP, Smith RA, et al. (2006) The effects of exogenous antioxidants on lifespan and oxidative stress resistance in Drosophila melanogaster. Mech Ageing Dev 127: 356-370.
- (1994) The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med 330: 1029-1035.
- Bjelakovic G, Nikolova D, Simonetti RG, Gluud C (2004) Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta-analysis. Lancet 364: 1219-1228.
- Heart Protection Study Collaborative Group (2002) MRC/BHF heart protection study of antioxidant vitamin supplementation in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet 360: 23-33.
- Pérez VI, Van Remmen H, Bokov A, Epstein CJ, Vijg J, et al. (2009) The overexpression of major antioxidant enzymes does not extend the lifespan of mice. Aging Cell 8: 73-75.
- Gašperlin M, Gosenca M (2011) Main approaches for delivering antioxidant vitamins through the skin to prevent skin ageing. Expert Opin Drug Deliv 8: 905-919.
- Lee HY, Choi CS, Birkenfeld AL, Alves TC, Jornayvaz FR, et al. (2010) Targeted expression of catalase to mitochondria prevents age-associated reductions in mitochondrial function and insulin resistance. Cell Metab 12: 668-674.
- Skulachev MV, Antonenko YN, Anisimov VN, Chernyak BV, Cherepanov DA, et al. (2011) Mitochondrial-targeted plastoquinone derivatives. Effect on senescence and acute age-related pathologies. Curr Drug Targets 12: 800-826.
- Shipounova IN, Svinareva DA, Petrova TV, Lyamzaev KG, Chernyak BV, et al. (2010) Reactive oxygen species produced in mitochondria are involved in age-dependent changes of hematopoietic and mesenchymal progenitor cells in mice. A study with the novel mitochondria-targeted antioxidant SkQ1. Mech Ageing Dev 131: 415-421.
- Pérez VI, Cortez LA, Lew CM, Rodriguez M, Webb CR, et al. (2011) Thioredoxin 1 overexpression extends mainly the earlier part of life span in mice. J Gerontol A Biol Sci Med Sci 66: 1286-1299.
- Qiu X, Brown K, Hirschey MD, Verdin E, Chen D (2010) Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab 12: 662-667.
- Falkenberg M, Larsson NG, Gustafsson CM (2007) DNA replication and transcription in mammalian mitochondria. Annu Rev Biochem 76: 679-699.
- Bua E, Johnson J, Herbst A, Delong B, McKenzie D, et al. (2006) Mitochondrial DNA-deletion mutations accumulate intracellularly to detrimental levels in aged human skeletal muscle fibers. Am J Hum Genet 79: 469-480.
- Hartmann N, Reichwald K, Wittig I, Dröse S, Schmeisser S, et al. (2011) Mitochondrial DNA copy number and function decrease with age in the short-lived fish Nothobranchius furzeri. Aging Cell 10: 824-831.
- Mao P, Gallagher P, Nedungadi S, Manczak M, Shirendeb UP, et al. (2012) Mitochondrial DNA deletions and differential mitochondrial DNA content in Rhesus monkeys: implications for aging. Biochim Biophys Acta 1822: 111-119.
- Greaves LC, Reeve AK, Taylor RW, Turnbull DM (2012) Mitochondrial DNA and disease. J Pathol 226: 274-286.
- Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, et al. (2004) Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429: 417-423.
- Trifunovic A, Hansson A, Wredenberg A, Rovio AT, Dufour E, et al. (2005) Somatic mtDNA mutations cause aging phenotypes without affecting reactive oxygen species production. Proc Natl Acad Sci U S A 102: 17993-17998.
- Ahlqvist KJ, Hämäläinen RH, Yatsuga S, Uutela M, Terzioglu M, et al. (2012) Somatic progenitor cell vulnerability to mitochondrial DNA mutagenesis underlies progeroid phenotypes in Polg mutator mice. Cell Metab 15: 100-109.
- Edgar D, Shabalina I, Camara Y, Wredenberg A, Calvaruso MA, et al. (2009) Random point mutations with major effects on protein-coding genes are the driving force behind premature aging in mtDNA mutator mice. Cell Metab 10: 131-138.
- Ghosh S, Lertwattanarak R, Lefort N, Molina-Carrion M, Joya-Galeana J, et al. (2011) Reduction in reactive oxygen species production by mitochondria from elderly subjects with normal and impaired glucose tolerance. Diabetes 60: 2051-2060.
- Kim EB, Fang X, Fushan AA, Huang Z, Lobanov AV, et al. (2011) Genome sequencing reveals insights into physiology and longevity of the naked mole rat. Nature 479: 223-227.
- Yu C, Li Y, Holmes A, Szafranski K, Faulkes CG, et al. (2011) RNA sequencing reveals differential expression of mitochondrial and oxidation reduction genes in the long-lived naked mole-rat when compared to mice. PLoS One 6: e26729.
- Houtkooper RH, Argmann C, Houten SM, Cantó C, Jeninga EH, et al. (2011) The metabolic footprint of aging in mice. Sci Rep 1: 134.
- Kil DY, Vester Boler BM, Apanavicius CJ, Schook LB, Swanson KS (2010) Age and diet affect gene expression profiles in canine liver tissue. PLoS One 5: e13319.
- Prahl S, Kueper T, Biernoth T, Wöhrmann Y, Münster A, et al. (2008) Aging skin is functionally anaerobic: importance of coenzyme Q10 for anti aging skin care. Biofactors 32: 245-255.
- Braidy N, Guillemin GJ, Mansour H, Chan-Ling T, Poljak A, et al. (2011) Age related changes in NAD+ metabolism oxidative stress and Sirt1 activity in wistar rats. PLoS One 6: e19194.
- Lee Y, Lee HY, Hanna RA, Gustafsson Ã…B (2011) Mitochondrial autophagy by Bnip3 involves Drp1-mediated mitochondrial fission and recruitment of Parkin in cardiac myocytes. Am J Physiol Heart Circ Physiol 301: H1924-1931.
- Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, et al. (2008) Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 27: 433-446.
- Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, et al. (2010) Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell 141: 280-289.
- Malena A, Loro E, Di Re M, Holt IJ, Vergani L (2009) Inhibition of mitochondrial fission favours mutant over wild-type mitochondrial DNA. Hum Mol Genet 18: 3407-3416.
- Chen H, McCaffery JM, Chan DC (2007) Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell 130: 548-562.
- Beregi E, Regius O (1987) Comparative morphological study of age related mitochondrial changes of the lymphocytes and skeletal muscle cells. Acta Morphol Hung 35: 219-224.
- Shaposhnikov VM (1985) The ultrastructural features of secretory cells of some endocrine glands in aging. Mech Ageing Dev 30: 123-142.
- Sato T, Tauchi H (1975) The formation of enlarged and giant mitochondria in the aging process of human hepatic cells. Acta Pathol Jpn 25: 403-412.
- Gilkerson RW, Selker JM, Capaldi RA (2003) The cristal membrane of mitochondria is the principal site of oxidative phosphorylation. FEBS Lett 546: 355-358.
- Lopes GS, Mora OA, Cerri P, Faria FP, Jurkiewicz NH, et al. (2004) Mitochondrial alterations and apoptosis in smooth muscle from aged rats. Biochim Biophys Acta 1658: 187-194.
- Kornmann B, Currie E, Collins SR, Schuldiner M, Nunnari J, et al. (2009) An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science 325: 477-481.
- Crompton M (2004) Mitochondria and aging: a role for the permeability transition? Aging Cell 3: 3-6.
- Tapia PC. Sublethal mitochondrial stress with an attendant stoichiometric augmentation of reactive oxygen species may precipitate many of the beneficial alterations in cellular physiology produced by caloric restriction, intermittent fasting, exercise and dietary phytonutrients: "mitohormesis" for health and vitality. Med Hypotheses 2006 66: 832-43.
- Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, et al. (2007) Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab 6: 280-293.
- SÃk A, Passer BJ, Koonin EV, Pellegrini L (2004) Self-regulated cleavage of the mitochondrial intramembrane-cleaving protease PARL yields Pbeta, a nuclear-targeted peptide. J Biol Chem 279: 15323-15329.
- Civitarese AE, MacLean PS, Carling S, Kerr-Bayles L, McMillan RP, et al. (2010) Regulation of skeletal muscle oxidative capacity and insulin signaling by the mitochondrial rhomboid protease PARL. Cell Metab 11: 412-426.
- Sripathi SR, He W, Atkinson CL, Smith JJ, Liu Z, et al. (2011) Mitochondrial-nuclear communication by prohibitin shuttling under oxidative stress. Biochemistry 50: 8342-8351.
- Guarente L (2008) Mitochondria--a nexus for aging, calorie restriction, and sirtuins? Cell 132: 171-176.
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, et al. (2009) Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325: 201-204.
- Sohal RS, Weindruch R (1996) Oxidative stress, caloric restriction, and aging. Science 273: 59-63.
- Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, et al. (2006) Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA 295: 1539-1548.
- Blanc S, Schoeller D, Kemnitz J, Weindruch R, Colman R, et al. (2003) Energy expenditure of rhesus monkeys subjected to 11 years of dietary restriction. J Clin Endocrinol Metab 88: 16-23.
- Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, et al. (2005) Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 310: 314-317.
- Selman C, Phillips T, Staib JL, Duncan JS, Leeuwenburgh C, et al. (2005) Energy expenditure of calorically restricted rats is higher than predicted from their altered body composition. Mech Ageing Dev 126: 783-793.
- Longo VD, Kennedy BK (2006) Sirtuins in aging and age-related disease. Cell 126: 257-268.
- Onyango P, Celic I, McCaffery JM, Boeke JD, Feinberg AP (2002) SIRT3, a human SIR2 homologue, is an NAD-dependent deacetylase localized to mitochondria. Proc Natl Acad Sci U S A 99: 13653-13658.
- Shi T, Wang F, Stieren E, Tong Q (2005) SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J Biol Chem 280: 13560-13567.
- Hafner AV, Dai J, Gomes AP, Xiao CY, Palmeira CM, et al. (2010) Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy. Aging (Albany NY) 2: 914-923.
- Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, et al. (2009) Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest 119: 2758-2771.
- Koyama T, Kume S, Koya D, Araki S, Isshiki K, et al. (2011) SIRT3 attenuates palmitate-induced ROS production and inflammation in proximal tubular cells. Free Radic Biol Med 51: 1258-1267.
- Safdar A, Bourgeois JM, Ogborn DI, Little JP, Hettinga BP, et al. (2011) Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice. Proc Natl Acad Sci U S A 108: 4135-4140.
- Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663-676.
- Suhr ST, Chang EA, Tjong J, Alcasid N, Perkins GA, Goissis MD, Ellisman MH, Perez GI, Cibelli JB. Mitochondrial rejuvenation after induced pluripotency. PLoS One 2010 Nov 23 5: e14095.
- Lapasset L, Milhavet O, Prieur A, Besnard E, Babled A, et al. (2011) Rejuvenating senescent and centenarian human cells by reprogramming through the pluripotent state. Genes Dev 25: 2248-2253.
- Stoll EA, Cheung W, Mikheev AM, Sweet IR, Bielas JH, et al. (2011) Aging neural progenitor cells have decreased mitochondrial content and lower oxidative metabolism. J Biol Chem 286: 38592-38601.
- Miyamoto K, Araki KY, Naka K, Arai F, Takubo K, et al. (2007) Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell 1: 101-112.
- Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, et al. (2006) Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med 12: 446-451.
- Mortensen M, Soilleux EJ, Djordjevic G, Tripp R, Lutteropp M, et al. (2011) The autophagy protein Atg7 is essential for hematopoietic stem cell maintenance. J Exp Med 208: 455-467.
- Rera M, Bahadorani S, Cho J, Koehler CL, Ulgherait M, et al. (2011) Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog. Cell Metab 14: 623-634.
- Durieux J, Wolff S, Dillin A (2011) The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell 144: 79-91.
Citation: Brown K, Liu Y, Chen D (2012) Aging: The Mitochondrial Connection. J Clin Exp Pathol S4:003. DOI: 10.4172/2161-0681.S4-003
Copyright: © 2012 Brown K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 16755
- [From(publication date): 0-2012 - Apr 08, 2025]
- Breakdown by view type
- HTML page views: 11908
- PDF downloads: 4847