Review Article Open Access
Accelerated Manufacturing of Large-Scale, Full-Length, Human-Like Glycosylated Antibodies for Bio-Defense
Christopher M Warner* and Matthew S Croughan
Amgen Bioprocessing Center, Keck Graduate Institute, Claremont, California, USA
- *Corresponding Author:
- Christopher M Warner
Amgen Bioprocessing Center
Keck Graduate Institute
Claremont, California, USA
Tel: 909.607.9267
E-mail: cwarner@kgi.edu
Received Date: October 11, 2012; Accepted Date: November 26, 2012; Published Date: November 29, 2012
Citation: Warner CM, Croughan MS,(2012) Accelerated Manufacturing of Large-Scale, Full-Length, Human-Like Glycosylated Antibodies for Bio-Defense. J Bioterr Biodef 3:122. doi:10.4172/2157-2526.1000122
Copyright: © 2012 Warner CM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Bioterrorism & Biodefense
Abstract
From the point of DNA sequence confirmation until production of meaningful clinical quantities of novel therapeutics, current manufacturing systems for many glycosylated proteins require several months of development. Consequently, in the event of mass-casualty epidemics, current systems will fail to provide sufficient and timely quantities of emergency medical counter measures. As the identity of many new biological threats are unlikely to be known in advance, pre-emptive manufacturing and stockpiling of countermeasures cannot always be performed. Preparedness for all biological catastrophes requires a radical solution to replace the current slow scale-up and manufacture of certain lifesaving medical countermeasures. Current clinical and commercial manufacturing methods for glycosylated proteins use stable cell lines for protein expression, wherein the gene coding for the protein of interest is stably integrated into the host cell genome. The generation, identification, banking, testing, and scale-up of suitable stable clones generally takes many months. Because this development time is not compatible with emergency manufacturing, an alternative method for rapid production of medical counter measure antibodies is needed. One such potential technique is transient gene expression. Transient gene expression is a common approach for production of research-grade antibodies. It is frequently
used to generate milligram to gram quantities of material within two to three weeks of DNA sequence confirmation. In the past, transient systems have been considered for emergency production of large quantities of antibodies, but dismissed due to low titers, high cost of DNA, uncertain regulatory environment, and the lack of sufficient, available manufacturing capacity. Recent developments, however, have substantially enhanced the viability of such an approach. This article will explore these developments and investigate the use of transient gene expression for rapid production of antibody-based medical countermeasures.
Recombinant protein production systems needed for catastrophic biological events
Despite advancements in the use of early detection systems, integrated epidemiologic surveillance, vaccination, and use of antimicrobial and antiviral agents, the final line of defense against a biological pathogen is the immune system of the exposed individual. Anti-microbial and anti-viral therapies may not work on all biological agents, especially resistant strains and toxin-based pathogens. Similarly, vaccination requires a window before protective responses are effective, often including multiple injections. Passive administration of immunotherapeutics, especially monoclonal antibodies (mAbs), can provide a state of immediate immunity that can last for weeks and possibly months [1,2].
The market demand for bio-defense has been defined, at least in one aspect, by the Defense Advanced Research Projects Agency (DARPA) through their Accelerated Manufacture of Pharmaceuticals (AMP) program [3]. As a starting point, these guidelines represent DARPA’s estimate of the production requirements for emergency medical counter measures sufficient to protect US military personnel (~3 million people). Compared to a civilian population, this quantity would translate to 1% of the total US population (~300 million), and would only be sufficient for initial clusters of disease outbreak, early responders, and military and police forces. Thus, this 3 million dose estimate defines a low end of the market demand for bio-defense medical counter measures, and provides metrics against which one can assess alternative capabilities.
The AMP program is a three phase system intended to build manufacturing capability for both vaccines and antibody recombinant proteins (r-proteins). An interested party could apply for Phase 1 funding, which requires production of either 12 kg of antibody or 1.2 g of vaccine within a 90 day period. If successful, the party could apply for Phase 2 funding wherein a 10x expansion of production capabilities would be necessary to generate 120 kg of antibody or 12 g of vaccine within a 90 day period. The final phase requires an additional 10x expansion of production capability to generate 1200 kg of antibody or 120 g of vaccine within 90 days (Table 1). Currently, there are three different partnerships attempting to meet vaccine production targets, all based upon subunit vaccines made through bacterial, fungal and plant technologies [4]. There has not been, however, any significant progress to date toward hitting the much more challenging antibody production targets.
| Phase | Antibody | Vaccine |
|---|---|---|
| 1 | 12 Kg | 1.2 g |
| 2 | 120 Kg | 12 g |
| 3 | 1200 Kg | 120 g |
Table 1: Milestones for bio-defense markets.
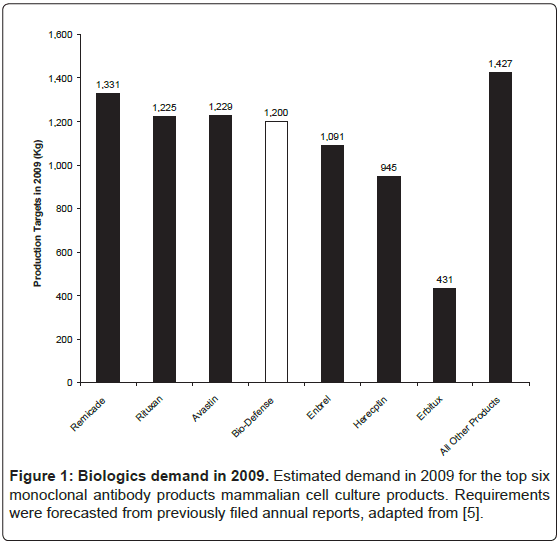
One of the reasons for the lack of success in rapid production of antibodies is an absence of private sector bio-manufacturing firms. This is, in part, due to difficult requirements in bio-defense markets. The Phase 3 production target of 1,200 kg of antibody is large compared to other biologic products and revenue streams are not predictable. The worldwide market for biologics in 2009 was $95 billion with 130 biologic products, which is 11% of the total pharmaceutical market and 16% larger compared to the previous year [5]. Of these 130 molecules, 27 biopharmaceutical products had sales in excess of $1 billion of which 17 were manufactured by mammalian cell culture. Nine of these were antibody-based products, including full length antibodies, antibody fragments, and Fc-fusion proteins. Of the full-length antibodies, only three have production targets larger than those in Phase 3 (Figure 1).
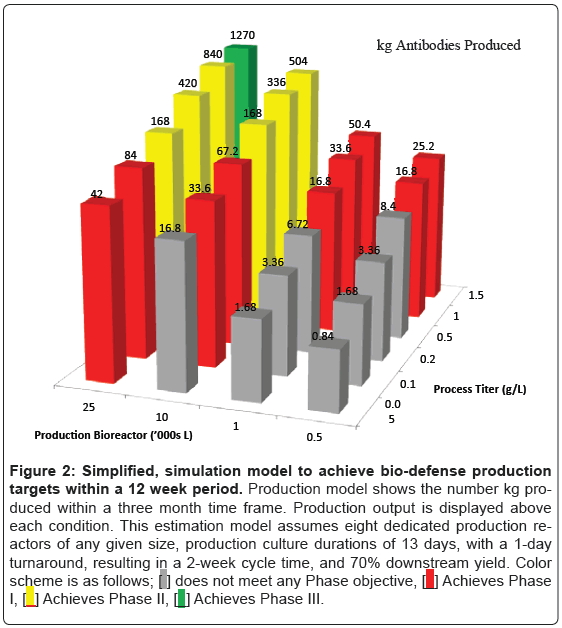
In order to estimate the possibility of manufacturing 1,200 kg of antibody material, a simplified, estimation model was constructed that evaluates manufacturing processes with a range of titers (50- 1500 mg/L) and a range of bioreactor sizes (500-25,000 L). Antibody production output is shown in Figure 2. This estimation model assumes eight dedicated production reactors of any given size, production culture durations of 13 days, with a 1-day turn around, resulting in a 2-week cycle time, and 70% downstream yield. This model identified a number of configurations that are able to satisfy Phase 1 and Phase 2 goals for antibody production. For instance, eight 25,000 L reactors with a final titer of 100 mg/L would be sufficient to satisfy Phase 2 targets of 120 kg. Similarly, a process with 1000 mg/L in eight 2,500 L bioreactors, which may consist of disposable bioreactors, would satisfy Phase 2 production targets. Phase 3 targets, however, would require a process with 1000 mg/L and a plant configuration of eight 25,000 L bioreactors. One such plant configuration, Genentech’s Vacaville II plant, is currently sitting idle and could provide sufficient capacity for bio-defense production [6]. Although not quite as large as the Vacaville II plant, there are additional large-scale antibody production plants sitting idle elsewhere in the US.
Figure 2: Simplified, simulation model to achieve bio-defense production
targets within a 12 week period. Production model shows the number kg produced
within a three month time frame. Production output is displayed above
each condition. This estimation model assumes eight dedicated production reactors
of any given size, production culture durations of 13 days, with a 1-day
turnaround, resulting in a 2-week cycle time, and 70% downstream yield. Color
scheme is as follows;  does not meet any Phase objective,
does not meet any Phase objective, Achieves Phase
I,
Achieves Phase
I,  Achieves Phase II,
Achieves Phase II,  Achieves Phase III.
Achieves Phase III.
Capacity within the biopharmaceutical industry
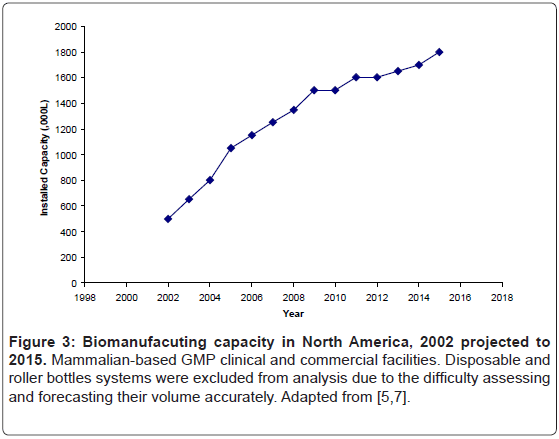
Employment of large scale bioreactors may be able to meet capacity requirements for bio-defense. Access to this capacity may occur in a number of fashions. One possibility would be through existing installed, stainless-steel bioreactors. The industry’s proclivity for these reactors increased available capacity over the last decade, and based upon current project already underway, will lead to further increase in capacity over, at least, the next few years (Figure 3) [7].
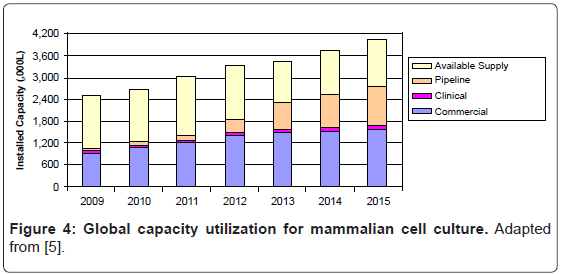
Unless a new use is found, such as for medical-counter measure production, large-scale stainless steel bioreactor capacity will likely be underutilized for several years. Levine found that only 69% of installed mammalian cell bioreactor capacity is currently being used (Figure 4) [5]. There are two primary reasons for this moderate bioreactor utilization rate.
First, there have been dramatic advances in cell culture productivity. Commercial cell culture titers have been doubling similar to Moore’s law, every 3.4 years [8]. Commercial cell culture processes for clinical production now have titers in the range of 4-10 g/L [9-11]. New, higher titer processes for established products are regularly approved and implemented for commercial production, resulting in reduced manufacturing plant time demands for those products.
Second, many firms adopt a risk adverse approach to managing capacity, due to both the skewed high cost of stocking out compared to the cost of carrying idle capacity, as well as the ethical considerations around potentially stocking out of a life-saving drug. Accordingly, many firms target the high end of market estimates, while assuming little or no gain in future titers, when they are sizing and designing manufacturing plants [12,13].
Due to an abundance of installed stainless steel bioreactors, biodefense production is possible within existing facilities. The 31% of capacity that is currently not being used within the industry could satisfy the demands of bio-defense under many scenarios.
Another option for bio-defense production is through the use of disposable bioreactors, or what is sometimes termed single-useteachnology (SUT). Because of an escalation of interest in SUTs, there have been significant advances in both disopable design and cost [14,15]. Compatible with nearly all aspects of antibody production, including upstream cultivation as well as downstream purification, SUTs are able to provide an end-to-end solution for biomanufacturing [16-18]. In addition, SUTs are able to be deployed in a variety of manufacturing locations, including military Forward Operating Bases (FOBs), which may include sterile warehouses, supply depots, or mobile manufacturing facilities. Such installations may result in onsite, on-demand manufacturing capabilities. With a plethora of possible capacity configurations, selection of a feasible cell culture process is essential. Selection of such a process includes determining an optimal host and expression system, which will be discussed below.
Antibodies require complex assembly
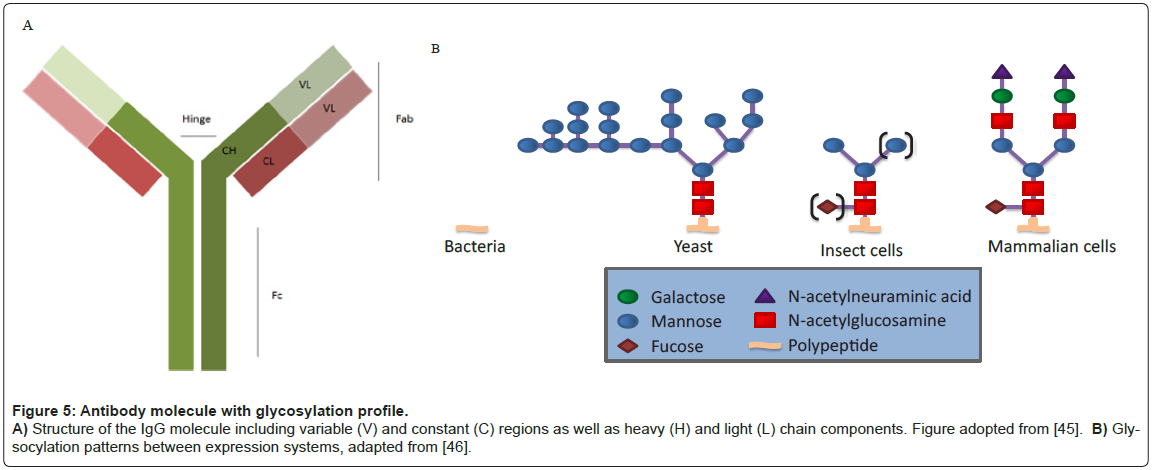
All human antibodies, or Immuno-Gamma globulins (IgG), contain a glycosylation site at an asparagine residue (N297) in the Fc domain (Figure 5) [19]. Other antibody isotypes often contain additional N-linked and O-linked glycans in the Fc domain and hinge region. In addition, 30% of human IgG antibodies contain N-linked glycosylation sites within the Fab domains, primarily attached within the variable regions (VH and/or VL) [20,21].
There are a number of ways these glycosylation moieties modulate both In vivo potency and In vitro stability of an antibody. The glycan at N297 in the Fc domain can affect protein resistance to proteolysis and the propensity to aggregate In vitro [22,23]. Early studies with human mouse chimeric IgG1 indicated that altered glycosylation can increase sensitivity to enzymatic digestion [24]. Moreover, certain glycan patterns confer greater protection to proteolysis than others [25,26]. Similar to enzymatic digestion, the thermal stability of full-length antibodies decreases when glycosylation is removed [27].
Glycosylation is also important for therapeutic efficacy. Upon antigen binding, antibodies form an immune complex which mediates the recruitment of complement proteins and triggers immune response signaling through Fc receptors (FcγRs) [28]. Antibodies destroy pathogenic cells through antibody-dependent cellular cytotoxicity (ADCC) and antibody dependent cell-mediated phagocytosis (ADCP). These mechanisms rely on the interaction between glycosylation of the antibody and the FcγRs [29-34]. Often, proper glycosylation is critical for therapeutic outcome.
Glycan heterogeneity in therapeutic antibodies is affected by many factors, including expression system host (i.e. mammalian, plant, yeast, and transgenic animals), the particular cell line (e.g., CHO vs. NSO), culture milieu, and downstream processing [35]. To date, all FDAapproved therapeutic antibodies have been produced in either CHO cells or in mouse myeloma cells (NS0 or SP2/0) [36]. Mammalian cell expression systems, especially CHO, have been preferred as their protein glycosylation machinery largely resembles those found in humans [37,38] and they can be cultured to high densities in chemically-defined medium. Along these lines, certain mammalian expression systems such as CHO are preferred as others may induce undesired immunogenicity in humans, or reduce therapeutic efficacy. For example, Cetuximab™ produced in the murine myeloma cell line SP2/0 is a chimeric mouse– human IgG1 monoclonal antibody that binds the epidermal growth factor receptor (EGFR). 22% of patients treated with Cetuximab™ had severe hypersensitivity reactions, characterized by human anti-mouse antibodies (HAMA) that were specific for the mouse produced glycan [39].
While mammalian systems are the preferred expression system for glycosylated antibodies, CHO cells are preferred over other mammalian expression systems. There has been significant progress in rapid manufacturing using Human Embryonic Kidney (HEK) cell lines because of a natural episomal system that maintains plasmids more effectively than other cell lines [40,41]. HEK, however, have been found to produce altered glyscoylation, while CHO have been shown to reproducibly produce human like glycosylation [42-44]. Therefore, the use of CHO cells as the expression system is preferred for the production of full-length, human like glycosylated antibodies for biodefense.
Anti-infective antibody success
Passive antibody preparations have been generated against a number of biological pathogens. These include many of the class A, B, and C agents, such as RSV, Vaccinia, Hepatits B and C, Dengue, HIV, Ebloa, Nipah, Incluenza, Bacillus anthracis and its toxin, Yersinia pestis, Clostridium botulinum neurotoxin, and many others [47,48]. Success of many of these molecules result from three inter-related characteristics. First, the antibody targets biologically relevant epitopes on the pathogen or toxin of interest with high affinity. Second, the antibody has a long serum half-life. Last, the antibody leads to effective pathogen clearance [49,50]. Antibody-mediated clearance of the antigen is typically associated with accumulation of the antibody-antigen complexes in the liver and/or spleen [51]. Neutralization of bacterial toxins has been shown to be dependent on maintaining antibody interaction with FcγRs through proper glycosylation [52]. In fact, one study demonstrated that an antibody preparation imparted significant protection against the botuliunum neurotoxin only when a full-length, human-glycosylated antibody was present. The authors hypothesized that the full- length antibody provided a clearance mechanism where toxin was removed from the system [53].
Rapid expression utilizing CHO
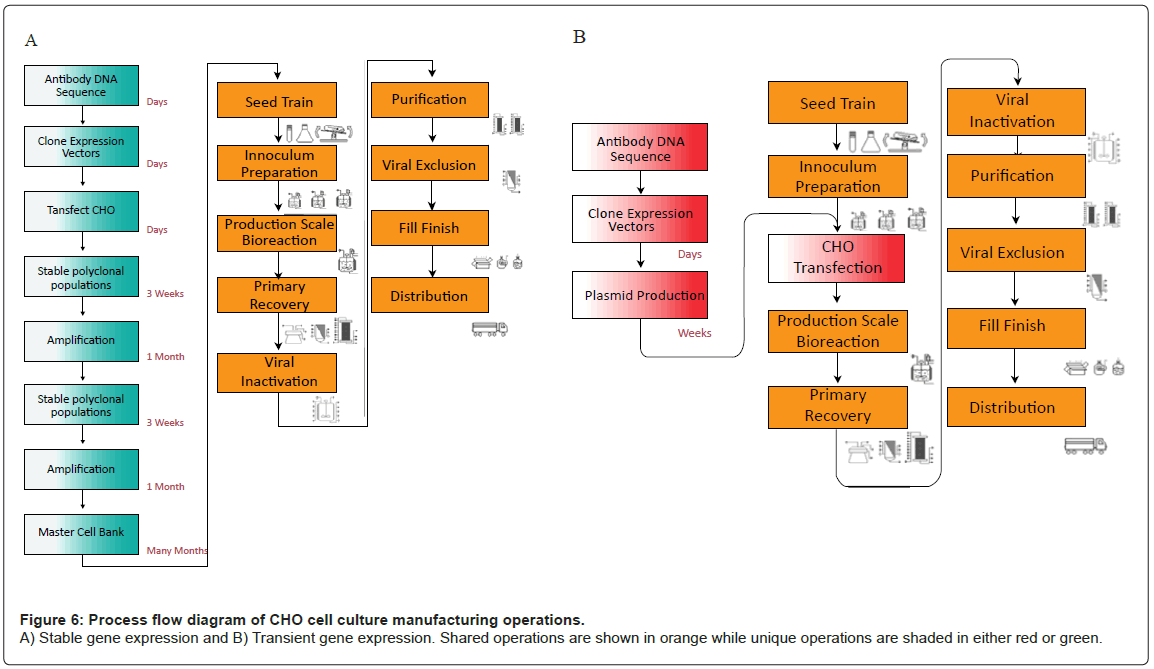
Commercial cell culture expression systems utilize stable gene expression (SGE) platforms to produce r-protein. SGE processes include identification of the antibody DNA sequence, which is then cloned into a plasmid expression vector and transfected into CHO cells where the plasmid is stably integrated in the host genome. Cells are subjected to multiple rounds of amplification wherein the gene of interest is amplified and specific productivities are increased up to 25-50 pg/cell-day or higher. Amplified cells are tested for stability and characterized through a battery of tests. Ultimately this strain selection process generates a clonal Master Cell Bank (MCB), which contains cells that will be used exclusively throughout commercial manufacture. Cells within the MCB typically have high rates of protein production, fast doubling times, and reproducible operating characteristics [54-56]. MCB generation is, however, a costly and lengthy process (from 4-12 months); (Figure 6A). Once the MCB is generated, inoculum is created by expanding cell mass through a series of cultures. Expanded inoculum is cultivated in a production bioreactor, where media formulation and culture conditions are optimized for r-protein production. R-proteins are subsequently purified through centrifugation, filtration and column chromatography. These purification operations remove host cell proteins, host cell DNA, endogenous retrovirus, and other impurities, creating the drug substance. The process culminates in a final vialing step, often called fill/finish to generate the final product [57,58].
Transient gene expression (TGE) utilizes the same downstream platform as SGE, but does not require the timely development of a master cell bank (Figure 6B). Instead of an MCB, TGE relies on cultivation of an aggregate pool of transfected CHO cells, in which the gene of interest is maintained extra-chromosomally. As plasmid DNA is not maintained throughout subsequent generations, new pools of transfectants must be created for each batch of production. TGE is, therefore much more rapid than SGE, but suffers from low specific productivities, and thus, overall titer. In addition, the high cost of DNA increases the cost of goods manufactured (COGM) of drug substance. Large scale production of plasmids prior to antibody manufacture may be a critical step in a TGE platform, but is out of scope of this article. A comparison of the two different systems (Table 2) demonstrates the advantages and disadvantages of each system.
| Transient expression | Stable expression |
|---|---|
| Expression of transfected genes are usually within 16-96 h, but expression is not propagated through subsequent generations | Heterologous DNA is integrated into the host genome and is stable throughout many generations |
| Transfection requires a large amount of plasmid DNA | Cell line development and testing requires an extensive period (4-12 months) |
| Expression level usually is relatively low (5-100 mg/L) |
After amplification and high producer selection, expression level is relatively high (1,000-10,000 mg/L) |
| Expression product is good for pre-clinical assessment, but no clear guidelines for clinical production | Stable cells lines can be used for large scale manufacturing leading to clinical trials and commercialization |
| It takes only a short period to expression of a product for “Proof of Principal” demonstration |
Table 2: Transient versus stable gene expression in mammalian cells.
Current state of transient gene expression
While transfection protocols have yet to be standardized, many components in the r-protein production process through TGE is similar to those found in SGE; including seed train expansion, harvest, purification, fill and finish [54]. Transient systems provide the same high quality protein, just much more quickly [40]. Several methods for gene transfer have been optimized for chemically defined transfection of animal cells, including both viral and non-viral methods.
Viral transient transfection includes the use of adeno-, alpha-, baculo-, lenti-, retro- and vaccina virus particles. Although these vehicles are very effective at delivering intact plasmid DNA into the nucleus, their use is limited due to limited DNA carrying capacity, lengthy processes for recombinant virus isolation, viral stock amplification and titration, bio-safety issues, and high cost of production [59]. Furthermore, viralbased processes are not allowed in any of the current large scale cell cultures plants, such as those built and operated by biopharmaceutical manufacturing firms. This is because it is difficult to validate cleaning, generating significant turn around time and increase in contamination risk for subsequent non-viral based production.
Non-viral methods for transient gene expression can be separated into mechanical and non-mechanical. Mechanical methods include microinjection, electroporation and others. These methods have been shown to have extremely favorable transfection efficiency, which is defined as the percentage of intact plasmid DNA actively transcribed in the nucleus. However, these methods are currently not viable for largescale cell culture because of both technical and economic reasons in their inability to scale above bench size.
Non-mechanical methods utilize calcium phosphate, cationic lipids, cationic polymers, and other nanoparticles, such as chitosan derivatives, to tightly condense DNA and form positively-charged complexes. Through electrostatic interaction with the negativelycharged cell membrane, DNA-complexes endocytose into the cytoplasm via endocytosis and eventually escape the endosomal vessicle. The DNA-complex can traffic through the cell’s cytoplasm into the nucleus, or transnucleate, through various pores on the nuclear envelope or, more commonly, through the breakdown of the nuclear membrane during mitosis. During this process the DNA-complex dissociates to allow for transgene expression. Transfection efficiency is low for nonmechanical methods due to the many physico-chemical barriers the cell has developed over thousands of years of evolution to prevent foreign DNA uptake [60]. However, transfection agents are believed to increase efficiency by facilitating the internalization into the cell and escape from endosomal/lysosomal vesicles, as well as protecting DNA from cytoplasmic nucleic acid degredation. Each of the various transfection agents has distinct advantages and limitations, discussed below.
Developed in the late 70’s, calcium phosphate was the first transfection agent used in mammalian cells and has been subsequently optimized up to the 100-L scale. Calcium phosphate’s dependence on serum for transfection, as well as buffer exchange steps posttransfection, limits its use in large scale cell culture [61]. Cationic lipid-based transfection (lipofection) has shown promise with high transfection efficiency; however, the cost of these compounds may exclude their use at large scale. Transfection methods employing cationic polymers seem to be the most actively used in the literature. Polyethelenimine (PEI) is currently the most widely used transfection agent because it is cost efficient, easy to handle and provides relatively high transfection efficiency as compared to alternative reagents [62].
PEI is available in various configurations with average molecular weight ranging from 2–750 kDa in either branched or linear forms. The 25 kDa linear PEI has been shown to be the optimal PEI for transfecting cells; its protocol and mechanism of delivery has been well studied [63,64]. Briefly, PEI, with a high cationic charge density, condenses DNA to form a compact positively-charged particle. This particle is endocytosed, and escapes endosomal vesicles through the proton-sponge effect, in which basic nitrogen atoms become protinated and cause an influx of chloride ions which rupture the vesicle through osmotic swelling [65,66]. Released plasmid DNA may transnucleate through complex methods mentioned above. Various cell types have been transfected using PEI at scales ranging from 1 ml to 1000 L. Over the years PEI has proven itself to be one of the most cost effective and efficient means of DNA delivery into mammalian cells.
Recent advances in TGE protocols
The considerable and ever-increasing number of publications on transient transfection technologies employed for r-protein production reflects the success of this approach over the past decade. Mirroring the advances in commercial cell culture, significant process development has improved TGE titers (Table 3). Media development, strain modification and optimization of transfection protocols have produced process that generates up to 1 g/L antibody quantities within two weeks [67]. Many of these approaches maintain cell viability while decreasing growth rates [68]. Both hypothermia and media supplements to induce gene expression are methods that decrease growth rates, extend viability and increase titer [41]. In addition, increased cell density during transfection results in both a greater utilization of DNA and produces cells with higher specific productivities.[69] Additional developments in strain engineering and process optimization will increase titers even higher.
| Expression (mg/L) | Cell | Process | Reference |
|---|---|---|---|
| 1000 | HEK 293* | Fed-batch, high density transfection with plasmid cocktail, hypothermia and gene expression additives | 67 |
| 300 | CHO-S | Batch transfection at moderate densities, optimized PEI/DNA/cell ratios, hypothermia | 70 |
| 90 | CHO DG44 | Use of valproic acid, hypothermia, moderate transfection densities | 71 |
| 80 | CHO K1SV | Fed-batch with DMSO and LiAC* | 42 |
| 80 | CHO-T | Episomal replication system, batch transfection at low density | 72 |
| 25 | DG44 | Low density transfection | 73 |
| 22 | DG44 | Disposable orbital bioreactors | 74 |
*HEK-293 cells are high producers, yet glycosylate unfavorably; DMSO (dimethyl sulfoxide); LiAC (Lithium Acetate)
Table 3: Antibody products produced with PEI-mediated TGE processes in mammalian cells.
Conclusions
Rapid manufacture of full-length, human-like glycosylated antibody based medical countermeasures is an unmet medical need. Because of improvements in titer, availability of installed stainless steel and SUT capacity, and improved process performance, TGE is a viable method to meet this need. Further investigation into the scaling of TGE production capabilities is necessary for an optimal bio-defense strategy.
References
- Casadevall A (2002) Passive antibody administration (immediate immunity) as a specific defense against biological weapons. Emerg Infect Dis 8: 833-841.
- Casadevall A (2005) Antibody-based defense strategies against biological weapons. American Society for Microbiology.
- Defense Advanced Research Projects Agency (DARPA) (2006) Accelerated manufacturing of pharmaceuticals. BAA06-31.
- Tether T (2008) Flexible manufacturing of pharmaceuticals for biological warfare defense. Subcommittee on Defense, Committee on Appropriations, United States House of Representatives.
- Levine HL (2010) A World of Biomanufacturing: Shortages or global glut? BioProcess Technology Consultants, Inc., BioProcess International Conference, Vienna, Austria.
- Reynolds EB (2010) Institutions, public policy and the product life cycle: The globalization of biomanufacturing and implications for Massachusetts. Industrial Performance Center Massachusetts Institute of Technology.
- Hunziker E (2009) Roche: on course through rough seas. Tokyo.
- Croughan M (2008) The silver anniversary of clinical protein production from recombinant CHO cells. ITQB.
- Omasa T, Onitsuka M, Kim WD (2010) Cell engineering and cultivation of chinese hamster ovary (CHO) cells. Curr Pharm Biotechnol 11:233-240.
- Hacker DL, De Jesus M, Wurm FM (2009) 25 years of recombinant proteins from reactor-grown cells - where do we go from here? Biotechnol Adv 27: 1023-1027.
- Jagschies G (2008) Where is biopharmaceutical manufacturing heading? BioPharm International 21: 72.
- Yang PY (2008) Bioproduction Workshop. Center for Biopharmaceutical Operations, University of California at Berkeley.
- Langer ES (2008) Managing biopharmaceutical manufacturing capacity in 2007, changes in demand and capacity projected through 2011. BioProcess Int.
- De Jesus M, Wurm FM (2011) Manufacturing recombinant proteins in kg-ton quantities using animal cells in bioreactors. Eur J Pharm Biopharm 78: 184-188.
- Langer ES (2009) Trends in single-use bioproduction, what users are saying. BioProcess Int.
- Eibl R, Kaiser S, Lombriser R, Eibl D (2010) Disposable bioreactors: the current state-of-the-art and recommended applications in biotechnology. Appl Microbiol Biotechnol 86: 41-49.
- Giroux D (2010) Single-use bioreactors with unparalleled mixing, scalability, and ease of use. Bioprocess Int 8: 130.
- DePalma A (2012) Single-use equipment on cusp of industrialization. Genetic Engineering & Biotechnology News 32.
- Jefferis R (2005) Glycosylation of recombinant antibody therapeutics. Biotechnol Prog 21: 11-16.
- Walsh G, Jefferis R (2006) Post-translational modification in the context of therapeutic proteins. Nat Biotechnol (24) 1241-1252.
- Liu H, Gaza-Bulseco G, Faldu D, Chumsae C, Sun J (2008) Heterogeneity of monoclonal antibodies. J Pharm Sci 97: 2426-2447.
- Wu SJ, Luo J, O'Neil KT, Kang J, Lacy ER, et al. (2010) Structure-based engineering of a monoclonal antibody for improved solubility. Protein Eng Des Sel 23: 643-651.
- Kayser V, Chennamsetty N, Voynov V, Forrer K, Helk B, et al. (2011) Glycosylation influences on the aggregation propensity of therapeutic monoclonal antibodies. Biotechnol J 6: 38-44.
- Wright A, Morrison SL (1997) Effect of glycosylation on antibody function: implications for genetic engineering. Trends Biotechnol 15: 26-32.
- Raju TS, Scallon BJ (2006) Glycosylation in the Fc domain of IgG increases resistance to proteolytic cleavage by papain. Biochem Biophys Res Commun 341: 797-803.
- Raju TS (2010) Impact of Fc glycosylation on monoclonal antibody effector functions and degredation by proteases. Current Trends in Monoclonal Antibody Development and Manufacturing Biotechnology: Pharmaceutical Aspects XI: 249-269.
- Mimura Y, Church S, Ghirlando R, Ashton PR, Dong S, et al. (2000) The influence of glycosylation on the thermal stability and effector function expression of human IgG1-Fc: properties of a series of truncated glycoforms. Mol Immunol 37: 697-706.
- Jefferis R (2009) Recombinant antibody therapeutics: the impact of glycosylation on mechanisms of action. Trends Pharmacol Sci 30: 356-362.
- Wright A, Tao MH, Kabat EA, Morrison SL (1991) Antibody variable region glycosylation: position effects on antigen binding and carbohydrate structure. EMBO J 10: 2717-2723.
- Solá RJ, Griebenow K (2010) Glycosylation of therapeutic proteins: an effective strategy to optimize efficacy. BioDrugs 24: 9-21.
- Krapp S, Mimura Y, Jefferis R, Huber R, Sondermann P (2003) Structural analysis of human IgG-Fc glycoforms reveals a correlation between glycosylation and structural integrity. J Mol Biol 325: 979-989.
- Jefferis R (2009) Glycosylation as a strategy to improve antibody-based therapeutics. Nat Rev Drug Discov 8: 226-234.
- Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, et al. (2009) Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood 113: 3716-3725.
- Beck A, Wagner-Rousset E, Bussat MC, Lokteff M, Klinguer-Hamour C, et al. (2008) Trends in glycosylation, glycoanalysis and glycoengineering of therapeutic antibodies and Fc-fusion proteins. Curr Pharm Biotechnol 9: 482-501.
- Sethuraman N, Stadheim TA (2006) Challenges in therapeutic glycoprotein production. Curr Opin Biotechnol 17: 341-346.
- Hossler P, Khattak SF, Li ZJ (2009) Optimal and consistent protein glycosylation in mammalian cell culture. Glycobiology 19: 936-949.
- Li H, d'Anjou M (2009) Pharmacological significance of glycosylation in therapeutic proteins. Curr Opin Biotechnol 20: 678-684.
- Verma R, Boleti E, George A (1998) Antibody engineering: comparison of bacterial, yeast, insect and mammalian expression systems. J Immunol Methods 216: 165-181.
- Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, et al. (2008) Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med 358: 1109-1117.
- Durocher Y, Perret S, Kamen A (2002) High-level and high-throughput recombinant protein production by transient transfection of suspension-growing human 293-EBNA1 cells. Nucleic Acids Res 30: E9.
- Geisse S, Fux C (2009) Recombinant protein production by transient gene transfer into Mammalian cells. Methods Enzymol 463: 223-238.
- Ye J, Kober V, Tellers M, Naji Z, Salmon P, et al. (2009) High-level protein expression in scalable CHO transient transfection. Biotechnol Bioeng 103: 542-551.
- Zeck A, Pohlentz G, Schlothauer T, Peter-Katalinic J, Regula JT (2011) Cell type-specific and site directed N-glycosylation pattern of FcγRIIIa. J Proteome Res 10: 3031-3039.
- Galbraith DJ, Tait AS, Racher AJ, Birch JR, James DC (2006) Control of culture environment for improved polyethylenimine-mediated transient production of recombinant monoclonal antibodies by CHO cells. Biotechnol Prog 22: 753-762.
- Peterson E, Owens SM, Henry RL (2006) Monoclonal antibody form and function: manufacturing the right antibodies for treating drug abuse. AAPS J 26: E383-E390.
- Jena BioSciences (2012) Macromolecular Crystallography. Catalong No 3.
- Reichert JM, Dewitz MC (2006) Anti-infective monoclonal antibodies: perils and promise of development. Nat Rev Drug Discov 5: 191-195.
- Berry JD, Gaudet RG (2011) Antibodies in infectious diseases: polyclonals, monoclonals and niche biotechnology. N Biotechnol 28: 489-501.
- Marks JD (2004) Deciphering antibody properties that lead to potent botulinum neurotoxin neutralization. Mov Disord 8: S101-S108.
- Reichert JM, Rosensweig CJ, Faden LB, Dewitz MC (2005) Monoclonal antibody successes in the clinic. Nat Biotechnol 23: 1073-1078.
- Daëron M (1997) Fc receptor biology. Annu Rev Immunol 15: 203-234.
- Abboud N, Chow SK, Saylor C, Janda A, Ravetch JV, et al. (2010) A requirement for FcγR in antibody-mediated bacterial toxin neutralization. J Exp Med 207: 2395-2405.
- Sepulveda J, Mukherjee J, Tzipori S, Simpson LL, Shoemaker CB (2010) Efficient serum clearance of botulinum neurotoxin achieved using a pool of small antitoxin binding agents. Infect Immun 78: 756-763.
- Wurm F, Bernard A (1999) Large-scale transient expression in mammalian cells for recombinant protein production. Curr Opin Biotechnol 10: 156-159.
- Chu L, Robinson DK (2001) Industrial choices for protein production by large-scale cell culture. Curr Opin Biotechnol 12: 180-187.
- Li F, Vijayasankaran N, Shen AY, Kiss R, Amanullah A (2010) Cell culture processes for monoclonal antibody production. MAbs 2: 466-479.
- Wurm FM (2004) Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol 22: 1393-1398.
- Kelley B (2007) Very large scale monoclonal antibody purification: the case for conventional unit operations. Biotechnol Prog 23: 995-1008.
- Varga CM, Tedford NC, Thomas M, Klibanov AM, Griffith LG, et al. (2005) Quantitative comparison of polyethylenimine formulations and adenoviral vectors in terms of intracellular gene delivery processes. Gene Ther 12: 1023-1032.
- Wiethoff CM, Middaugh CR (2003) Barriers to nonviral gene delivery. J Pharm Sci 92: 203-217.
- Jordan M, Schallhorn A, Wurm FM (1996) Transfecting mammalian cells: optimization of critical parameters affecting calcium-phosphate precipitate formation. Nucleic Acids Res 24: 596-601.
- Fischer D, Bieber T, Li Y, Elsässer HP, Kissel T (1999) A novel non-viral vector for DNA delivery based on low molecular weight, branched polyethylenimine: effect of molecular weight on transfection efficiency and cytotoxicity. Pharm Res 16: 1273-1279.
- Boussif O, Lezoualc'h F, Zanta MA, Mergny MD, Scherman D, et al. (1995) A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA 92: 7297-7301.
- Bertschinger M, Backliwal G, Schertenleib A, Jordan M, Hacker DL, et al. (2006) Disassembly of polyethylenimine-DNA particles in vitro: implications for polyethylenimine-mediated DNA delivery. J Control Release 116: 96-104.
- Akinc A, Thomas M, Klibanov AM, Langer R (2005) Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. J Gene Med 7: 657- 663.
- Thomas M, Klibanov AM (2003) Non-viral gene therapy: polycation-mediated DNA delivery. Appl Microbiol Biotechnol 62: 27-34.
- Backliwal G, Hildinger M, Chenuet S, Wulhfard S, De Jesus M, et al. (2008) Rational vector design and multi-pathway modulation of HEK 293E cells yield recombinant antibody titers exceeding 1 g/l by transient transfection under serum-free conditions. Nucleic Acids Res 36: e96.
- Geisse S (2009) Reflections on more than 10 years of TGE approaches. Protein Expr Purif 64: 99-107.
- Baldi L, Hacker DL, Adam M, Wurm FM (2007) Recombinant protein production by large-scale transient gene expression in mammalian cells: state of the art and future perspectives.Biotechnol Lett 29: 677-684.
- Rajendra Y, Kiseljak D, Baldi L, Hacker DL, Wurm FM (2011) A simple high-yielding process for transient gene expression in CHO cells. J Biotechnol 153: 22-26.
- Wulhfard S, Baldi L, Hacker DL, Wurm F (2010) Valproic acid enhances recombinant mRNA and protein levels in transiently transfected Chinese hamster ovary cells. J Biotechnol 148: 128-132.
- Codamo J, Hou JJC, Hughes BS, Gray PP, Munro TP (2011) Efficient mAb production in CHO cells incorporating PEI-mediated transfection, mild hypothermia and the co-expression of XBP-1. J Chem Technol Biotechnol 86: 923-934.
- Pham PL, Kamen A, Durocher Y (2006) Large-scale transfection of mammalian cells for the fast production of recombinant protein. Mol Biotechnol 34: 225-237.
- Muller N, Derouazi M, Van Tilborgh F, Wulhfard S, Hacker DL, et al. (2007) Scalable transient gene expression in Chinese hamster ovary cells in instrumented and non-instrumented cultivation systems. Biotechnol Lett 29: 703-711.
Relevant Topics
- Anthrax Bioterrorism
- Bio surveilliance
- Biodefense
- Biohazards
- Biological Preparedness
- Biological Warfare
- Biological weapons
- Biorisk
- Bioterrorism
- Bioterrorism Agents
- Biothreat Agents
- Disease surveillance
- Emerging infectious disease
- Epidemiology of Breast Cancer
- Information Security
- Mass Prophylaxis
- Nuclear Terrorism
- Probabilistic risk assessment
- United States biological defense program
- Vaccines
Recommended Journals
Article Tools
Article Usage
- Total views: 16101
- [From(publication date):
November-2012 - Nov 24, 2024] - Breakdown by view type
- HTML page views : 11472
- PDF downloads : 4629