Review Article Open Access
Abnormal Brain Circuitry and Neurophysiology Demonstrated by Molecular Imaging Modalities in Schizophrenia
Ayden Jacob1*, Sharon Cohen2and Abass Alavi31Oxford Institute for Radiation Oncology; University of Oxford, UK
3Department of Nuclear Medicine, University of Pennsylvania, USA
- Corresponding Author:
- Ayden Jacob
Oxford Institute for Radiation Oncology
University of Oxford, UK
E-mail: aydenjacob@berkeley.edu
Received date: June 03, 2013; Accepted date: June 25, 2013; Published date: June 27, 2013
Citation: Jacob A, Cohen S, Alavi A (2013) Abnormal Brain Circuitry and Neurophysiology Demonstrated by Molecular Imaging Modalities in Schizophrenia. J Alzheimers Dis Parkinsonism 3: 114. doi: 10.4172/2161-0460.1000114
Copyright: © 2013 Jacob A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Schizophrenia is a major mental disorder characterized by functional impairment and the presentation,
persistence, and severity of symptoms. Characterized as a brain disorder, the etiology of schizophrenia remains elusive. Nevertheless, neuroimaging modalities have advanced the understanding of the neuroscientific community in regards to the structural and functional abnormalities inherent in the brains of schizophrenics. Neurochemical and molecular strategies are beginning to form a subtle consensus in the biomedical community as to the specificities of the disease on an organic level. Developing our understanding of the micro-abnormalities present in this disorder will allow the medical community to advance our neuropharmological approach to treating, curing, and perhaps preventing the onset of this disabling psychiatric disease.
Keywords
Neuroimaging; Neuropsychiatry; Mental health; Schizophrenia
Introduction
Commonly manifested as auditory hallucinations, paranoid and bizarre delusions, and disorganized speech and thinking, schizophrenia is a serious mental disorder affecting a person�s thinking, emotions and behavior. Patients report hearing voices that may tell them what to do, as well as demonstrate a lack of responsiveness, loss of motivation and severe impairment in social cognition; furthermore, patients may be described as speaking in a �word salad� as their auditory and linguistic capabilities may be impaired. Schizophrenia is a severe psychiatric disorder that affects 1% of the population and is one of the top ten causes of disability worldwide [1]. Usual onset begins in late adolescence or early adulthood and is characterized by positive psychotic symptoms, such as delusions and hallucinations and disorganized speech, as well as negative psychotic symptoms which include emotional blunting and loss of drive. These are accompanied by cognitive impairments, particularly in memory and executive functions, in addition to significant social and occupational dysfunction [2].
A core pathophysiological abnormality that could account for all the different clinical features of the disorder has not been identified. Diagnoses of these disorders are based on self-reported experiences of the patient, as well as abnormal behavior reported by others. Evidently, there are many psychiatric illnesses which contain a similar range of psychotic symptoms, such as bipolar disorder, major depression, drug intoxication, and drug-induced psychosis. Thus, diagnosing patients specifically for the range of schizophrenic disorders is complex and must be done thoroughly and diligently, in order to rule out any other possibilities. The most widely used diagnostic criteria based upon the Diagnostic and Statistical Manual requires patients to have had certain combinations of symptoms continuously for at least six months [3]. However, even when detailed criteria are used in conjunction with standardized diagnostic interviews, there is still significant variability between clinicians in diagnosing the disorder [4]. A neurobiological feature which may be present as a common denominator might allow the diagnostic criteria for schizophrenia to be more clearly understood, both by physicians and patients alike. In addition, the utilization of various neuroimaging modalities to detect the structural and functional abnormalities in the brains of psychiatric patients can pave the way for the future of neuropsychiatric treatment and prognosis. This article reviews the current literature regarding the use of neuroimaging techniques in the evaluation, diagnosis, and treatment of schizophrenia [5,6].
Genesis Debate
A continuous argument has developed as to whether the origins of schizophrenia are neurodegenerative or neurodevelopmental. Buckley [7] ascribe the origins of schizophrenia to a neurodevelopmental view based on neuroimaging studies which reveal structural changes in the brain due to early childhood noxious events that are either genetic or environmental, or involve an interrelated combination of both nature and nurture. Although the neurodevelopmental view was accepted for the conceptual understanding of the onset and cause of schizophrenia, significant evidence presented by Buckley presents a neurodegenerative hypothesis in patients with schizophrenia [7]. Neuroimaging studies have followed the course of brain changes in schizophrenic brains and show a deterioration in brain structure with compelling evidence now of a loss of cortical tissue and progressive ventricular enlargement. These findings have led some to recast the neurodevelopment versus neurodegeneration dichotomy essentially as a two-stage pathogenic trajectory [8]. From this biomedical perspective, it is proposed that some deleterious event causes primal neurodevelopmental cortical impairment. This impaired cortical substrate confers susceptibility thereafter to the later emergence of neurodegenerative processes [7]. Consequently, the neuroscientific community has illustrated that schizophrenia may represent an interactive process between neurodevelopmental vulnerability and progressive deterioration with neurotoxicity [9]. According to Lindenberg, convergent evidence shows that the disease process of schizophrenia long precedes the manifest illness, and abnormalities found in patients may reflect a complex and advanced condition that could be too late in the trajectory of the disease for efficient treatment [10]. Future neuroimaging studies will be necessary in order to best determine the relationship between these two primary processes in the development of schizophrenia.
Neuroanatomical Abnormalities
In schizophrenia, anatomical imaging has highlighted decreases in grey matter, white matter, and whole brain matter volumes along with an increase in ventricular volume. Such studies performed primarily with magnetic resonance imaging can accurately quantify brain volumes. Chronic schizophrenics present with more extensive volume reductions in the cortex, specifically in the medial and left dorsolateral prefrontal cortex, as well as in the left superior temporal gyrus [11]. Further imaging data has correlated schizophrenic brains with decreased volumes in the hippocampus, thalamus and left amygdala region, as along with the bilateral insula and anterior cingulated [11]. Interestingly, hippocampal volume is also reduced in relatives of schizophrenic patients [12] suggesting a potential genetic and heritable component to the disease.
Microstructure Brain Abnormalities
There exists an inherent connection between the macroscopic structural abnormalities found in the brains of schizophrenic patients and the neuronal microcircuits in those very domains [13]. Specifically, pyramidal neurons of the prefrontal cortex are the main source of excitatory corical-cortical neurotransmission and display a reduced size in schizophrenics. Further, pyramidal neurons are also packed more densely in these brains, which indicates a reduction in dendritic spines and axon terminals which occupy the space between neurons. It is hypothesized that this may be a consequence of intense synaptic pruning during adolescence. Interneuron populations are also adversely affected in the brains of schizophrenics. Interneurons in the prefrontal cortex which contain parvalbumin display consistent signs of reduced GABAergic neural transmission [14]. In the hippocampus, cell bodies of pyramidial neurons are smaller and dendritic spines are reduced. Several studies have indicated that in the thalamus there is a reduction in the quantity of neurons, specifically in the mediodorsal nucleus and pulvinar [15]. Collectively, these microstructure abnormalities correlate well with neuroimaging findings on the structural scale. The molecular abnormalities indicate local processing dysfunction, especially in glutamatergic drive to GABAergic parvalbumin containing interneurons and intracortical connectivity. Further, these findings suggest changes in long-range connectivity, including important thalamic afferents. Prefrontal cortex modulation is highly influenced by the dopamanergic system in the brain. Thus, the delicate balance between information maintenance and flexible adjustment of information that characterizes executive function depends quite critically on an optimal level of dopamanergic signalling. This signalling system interacts with the prefrontal cortex from the midbrain and ventral striatum. Schizophrenic brains demonstrate reduced prefrontal inputs, thereby facilitating their significant symptoms on the phenotypic scale.
Neuronal Circuitry Abnormalities
Neuroimaging has been useful in defining abnormal circuits, especially within the dorsolateral prefrontal cortex, and has shown that, rather than a uniform disruption or disconnectivity, schizophrenia is characterized by �dysconnectivity.� Although no accepted definition has been put forth, dysconnectivity within the microcircuitry of brain processing may be explained by functional interactions which are altered in a regionally and functionally differentiated manner. During working memory, dorsolateral prefrontal cortex connectivity is altered in patients with schizophrenia and subjects at risk [16]. Interhemispheric prefrontal connectivity is reduced in patients and relatives, whereas a dysfunctional increase in the connectivity with the hippocampal formation has been observed in chronic and first episode patients [17]. It is applicable to the phenotype of schizophrenics that the hippocampal formation provides input to the dorsolateral prefrontal cortex, and neonatal hippocampal formation lesions in animals induce prefrontal cortex abnormalities [18], indicating a causal role of the interaction between these two regions in schizophrenia. This frontohippocampal dysconnection hypothesis is also as also supported by the notion that the hippocampal formation is selectively vulnerable to some early neurodevelopmental disturbances. Further, multiple parallel interactions between the prefrontal cortex, thalamus, and striatum form feedback loops which are essential for basic information processing. Feedback loops are disturbed in schizophrenia patients [19]. This prefrontal-neostriatal system is modulated by midbrain dopaminergic neurons which project to the cortex and striatum and are subsequently regulated by prefrontal cortex efferent neurons [20]. Collectively, the structural and molecular abnormalities discovered via advanced biophysical imaging technologies illustrate an intricate system in the brain of a schizophrenic which performs its tasks in a dehabilitating manner.
Psychiatric Neuroimaging Technologies
As a result of rapid technological development in recent years, a range of functional imaging techniques is now available for the assessment in vivo of human brain function [5]. Positron emission tomography (PET), single photon emission computed tomography (SPECT) and functional magnetic resonance imaging (fMRI) provide both temporal and spatial information that can be used to localize regional brain activity during the resting state or precisely controlled cognitive conditions. These techniques have considerably advanced our understanding of human brain function and the pathophysiology of schizophrenia [5]. Molecular neuroimaging studies permit the examination of chemical changes in the brain related to schizophrenia. Positron emission tomography and single-photon emission tomography employ radioactive tracers to generate images reflecting the distribution of ligands for specific molecules in the brain, and they can be used to study the synthesis and release of neurotransmitters and the availability of neurotransmitter receptors [6]. PET and SPECT have had a considerable impact on molecular neuropharmacology, enabling in vivo assessment in humans of the level of receptor availability in schizophrenia, and also the level of receptor occupancy by antipsychotic medications at doses leading to clinical efficacy and treatment side-effects. These studies have been essential in developing current theories about the neurobiology of schizophrenia. Functional magnetic resonance imaging and other imaging techniques that measure regional cerebral blood flow have demonstrated that resting neural activity and activation during a variety of cognitive tasks are abnormal in several brain areas in schizophrenia [6]. A variety of Neuromolecular and neurochemicals imaging techniques are utilized in the diagnosis of schizophrenia, as brain function abnormalities may be detected efficiency by these advanced biomedical instruments (Figure 1).
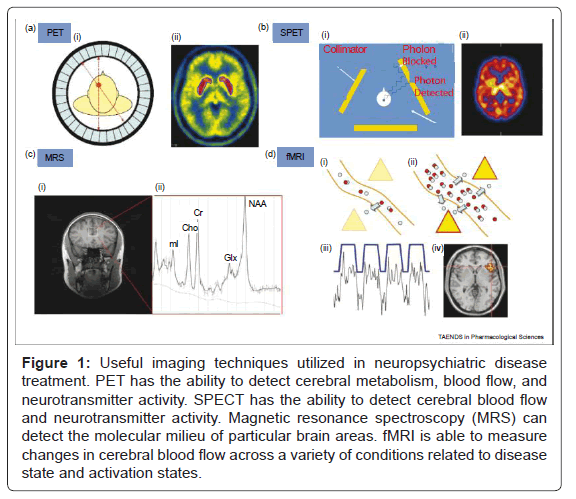
Figure 1: Useful imaging techniques utilized in neuropsychiatric disease treatment. PET has the ability to detect cerebral metabolism, blood flow, and neurotransmitter activity. SPECT has the ability to detect cerebral blood flow and neurotransmitter activity. Magnetic resonance spectroscopy (MRS) can detect the molecular milieu of particular brain areas. fMRI is able to measure changes in cerebral blood flow across a variety of conditions related to disease state and activation states.
PET ligands emit positrons which generate a pair of photons that travel in diametrically opposite directions, whereas SPET ligands emit a single photon. The scanners contain detectors positioned around the head that are sensitive to photons released from the radiotracer. Information from the detectors is used to construct an image that shows the distribution of the tracer in the brain. Magnetic resonance spectroscopy (MRS) measures the relative concentration of different chemicals in the brain. When exposed to a magnetic field, chemicals respond differently according to the number and location of protons in their molecular structure. Within a region of interest in the brain, MRS exploits this property to yield a spectrum of peaks (Figure 1) corresponding to the different chemicals present. MRS can thus be used to estimate the local concentration of glutamate and glutamine (Glx), N-acetyl aspartate (NAA), creatine (Cr), myoinositol (mI) and choline (Cho). fMRImeasures the BOLD (blood oxygenation level dependent) response. An increase in neural activity in response to an experimental stimulus increases local blood flow to provide more oxygen (blue circles), delivered as oxyheamoglobin (red and blue circles together). Blood flow during normal neural activity can be observed (Figure 1), as well as areas of increased or decreased perfusion during specific activation tasks (Figure 1). Oxy- and deoxyhemoglobin have different paramagnetic qualities and the local change in their ratio results in signal that can be detected by the MRI scanner. fMRI has been the primary modality used in the field of cognitive neuroscience to elucidate the areas of the brain involved in different cognitive and emotional processes.
PET Imaging in Schizophrenia
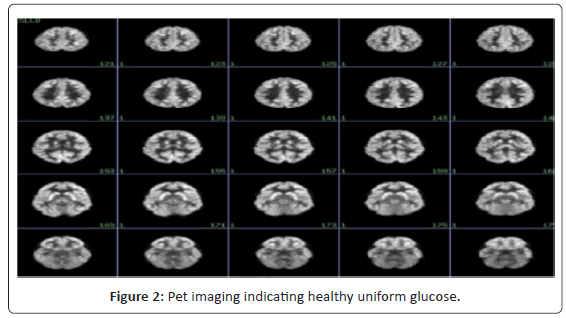
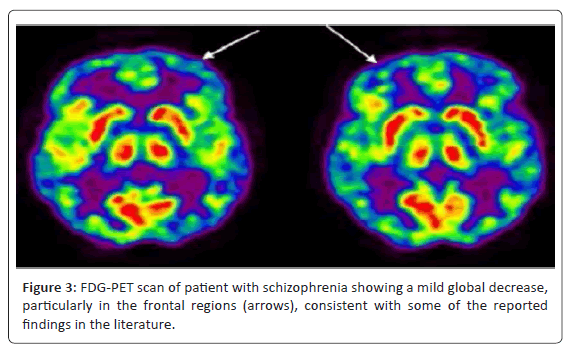
Positron emission tomography (PET) has provided substantial contributions to understanding the neurophysiological basis of neurological and psychiatric disorders. Studies of cerebral blood flow and metabolism have provided insights into the pathology and treatment of these complex diseases. Neurotransmitter studies using PET imaging have further elucidated pathophysiologic mechanisms involved. The currently available tracer for clinical brain PET is [18F] fluorodeoxyglucose (FDG), which measures cerebral glucose metabolism. Usually, these studies are performed with the subject receiving an intravenous injection at rest, allowing an uptake period of about 30 min, and then scanning for about 30 min. PET imaging has contributed to our understanding of the disease processes and their associated pathophysiology. Functional abnormalities in schizophrenia have been clearly demonstrated using PET imaging [21,22] (Figure 2 and 3).
Molecular imaging studies in schizophrenia are consistent with the notion that dopamine abnormalities are a key pathophysiological feature of the disorder. Studies indicate that schizophrenia is associated with increased pre-synaptic striatal dopamine synthesis and storage [23,24] and increased striatal release of dopamine following amphetamine administration [25,26]. In addition to dopamine abnormalities, theories of schizophrenia indicate other neurotransmitters which may be aberrantly affected. Krystal et al. and Newcomer et al. have reported that when healthy individuals take uncompetitive antagonists for the NMDA (N-methyl-D-aspartate) receptor (such as PCP (phencyclidine) or ketamine) they develop transient positive and negative psychotic symptoms and cognitive impairments that resemble those of schizophrenia. This is significant because NMDA receptor blockade on GABAergic interneurons can lead to a disinhibition of glutamatergic projection neurons and elevated glutamate release. It is hypothesized that similar mechanisms could occur in schizophrenia, through dysfunction of NMDA receptors or the GABAergic interneurons upon which they are expressed. Elevated glutatame as a potential pathophysiological mechanism in schizophrenics is useful as it may contribute to the reductions in grey matter volume seen in MRI studies of schizophrenics [27].
Several PET studies have been performed to determine whether or not left hemispheric dysfunction can be detected in schizophrenics. In some studies, patients with schizophrenia at rest had increased perfusion and metabolism in the left hemispheric cerebral cortex relative to the right. Also, the severity of the symptoms of schizophrenia correlated with the degree of hyperactivation of the left hemisphere and not with the degree of hypofrontality. This concurs with a study by Sheppard and colleagues, which found increased blood flow to the left hemisphere using 15O-H2O PET. Early and colleagues found increased CBF in the left globus pallidus in patients with schizophrenia.
Corticostriatal Function and PET
Corticcobasal ganglia neuronal circuitry is of elementary importance for most motor and mental behavior. The neuronal activity of the corticostriatal circuits is significantly dependent on the concentration and affects of dopamine. Consequently, the dopmanergic systems play a vital role in corticostriatal function. Evidently, increased capacity in the basal ganglia for dopamine synthesis in patients with schizophrenia has been reported extensively by the scientific community. These important findings concur with other PET studies which demonstrate elevated psychostimulant evoked dopamine release in the benzamide competitive binding paradigm [28]. Collectively, these findings may support the underlying hypothesis which correlates the positive symptoms of schizophrenia with an inherent functional excess of dopamine [29]. Kawagoe et al. report neurophysiological studies of dopaminergic neurons which demonstrate that changes in this particular firing pathway may influence excitability in the corticobasal ganglia pathways [30]. Schultz further elaborate that these neuronal circuitry deficiencies are particularly evident in the prediction of temporal errors in reward [31]. Consequently, dopaminergic neuronal firing alterations may significantly influence important behavioral patterns which are of limited function in schizophrenic patients. Hietla et al. [32] and Reith et al. [33] report studies which utilized PET imaging instrumentation to demonstrate that unmedicated patients with schizophrenia contain an increase in dopamine synthesis rate when compared to healthy controls. Kumakura et al. concluded from their studies with PET in schizophrenic patients that a significant difference in patterns of brain dopamine utilization separates schizophrenia from the healthy state [34].
Conclusion
The association of deficits in executive cognitive functions with reduced functions of a frontal-cortical-based cognitive control system in the brain has important implications for both the pathophysiology of cognitive pathology in the illness as well as for the development of therapies targeting this disabling aspect of the disease. Neuroimaging, particularly PET and SPECT with their array of radiotracers, is used to study many physiological and pathological states in the brain. In order to continue to advance our understanding of schizophrenia, its causes and treatments, future neuroimaging studies will be necessary to assess metabolism, blood flow, and neuromolecular activity.
Acknowledgement
NIH Funding Code: S10RR020976
References
- Lopez AD, Murray CC (1998) The global burden of disease, 1990-2020. Nat Med 4: 1241-1243.
- Kurtz MM (2005) Neurocognitive impairment across the lifespan in schizophrenia: an update. Schizophr Res 74: 15-26.
- American Psychiatric Association (1994) Diagnostic and Statistical Manual for Mental Disorders (DSM-IV), American Psychiatric Association.
- Fusar-Poli P, Perez J, Broome M, Borgwardt S, Placentino A, et al. (2007) Neurofunctional correlates of vulnerability to psychosis: a systematic review and meta-analysis. Neurosci Biobehav Rev 31: 465-484.
- Garry D Honey, Edward T Bullmore (2002) Functional Neuroimaging and Schizophrenia. Research Aspects in Schizophrenia 26-30.
- McGuire P, Howes OD, Stone J, Fusar-Poli P (2008) Functional neuroimaging in schizophrenia: diagnosis and drug discovery. Trends Pharmacol Sci 29: 91-98.
- Buckley PF (2005) Neuroimaging of schizophrenia: structural abnormalities and pathophysiological implications. Neuropsychiatr Dis Treat 1: 193-204.
- Church SM, Cotter D, Bramon E, Murray RM (2002) Does schizophrenia result from developmental or degenerative processes? J Neural Transm Suppl : 129-147.
- Mathalon DH, Sullivan EV, Lim KO, Pfefferbaum A (2001) Progressive brain volume changes and the clinical course of schizophrenia in men: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry 58: 148-157.
- Meyer-Lindenberg A (2010) From maps to mechanisms through neuroimaging of schizophrenia. Nature 468: 194-202.
- Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E (2008) The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry 165: 1015-1023.
- Goldman AL, Pezawas L, Mattay VS, Fischl B, Verchinski BA, et al. (2008) Heritability of brain morphology related to schizophrenia: a large-scale automated magnetic resonance imaging segmentation study. Biol Psychiatry 63: 475-483.
- Boos HB, Aleman A, Cahn W, Hulshoff Pol H, Kahn RS (2007) Brain volumes in relatives of patients with schizophrenia: a meta-analysis. Arch Gen Psychiatry 64: 297-304.
- Lewis DA, Sweet RA (2009) Schizophrenia from a neural circuitry perspective: advancing toward rational pharmacological therapies. J Clin Invest 119: 706-716.
- Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, et al. (2003) Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci 23: 6315-6326.
- Byne W, Hazlett EA, Buchsbaum MS, Kemether E (2009) The thalamus and schizophrenia: current status of research. Acta Neuropathol 117: 347-368.
- Wolf RC, Vasic N, Sambataro F, Hose A, Frasch K, et al. (2009) Temporally anticorrelated brain networks during working memory performance reveal aberrant prefrontal and hippocampal connectivity in patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 33: 1467�1473.
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, et al. (2009) Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A 106: 1279-1284.
- Crossley NA, Mechelli A, Fusar-Poli P, Broome MR, Matthiasson P, et al. (2009) Superior temporal lobe dysfunction and frontotemporal dysconnectivity in subjects at risk of psychosis and in first-episode psychosis. Hum Brain Mapp 30: 4129-4137.
- Bertolino A, Saunders RC, Mattay VS, Bachevalier J, Frank JA, et al. (1997) Altered development of prefrontal neurons in rhesus monkeys with neonatal mesial temporo-limbic lesions: a proton magnetic resonance spectroscopic imaging study. Cereb Cortex 7: 740-748.
- Braff DL, Geyer MA (1990) Sensorimotor gating and schizophrenia. Human and animal model studies. Arch Gen Psychiatry 47: 181-188.
- Jaskiw GE, Karoum FK, Weinberger DR (1990) Persistent elevations in dopamine and its metabolites in the nucleus accumbens after mild subchronic stress in rats with ibotenic acid lesions of the medial prefrontal cortex. Brain Res 534: 321-323.
- Sedvall G (1992) The current status of PET scanning with respect to schizophrenia. Neuropsychopharmacology 7: 41-54.
- Cleghorn JM, Zipursky RB, List SJ (1991) Structural and functional brain imaging in schizophrenia. J Psychiatry Neurosci 16: 53-74.
- Abi-Dargham A, Gil R, Krystal J, Baldwin RM, Seibyl JP, et al. (1998) Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. Am J Psychiatry 155: 761-767.
- Lindström LH, Gefvert O, Hagberg G, Lundberg T, Bergström M, et al. (1999) Increased dopamine synthesis rate in medial prefrontal cortex and striatum in schizophrenia indicated by L-(beta-11C) DOPA and PET. Biol Psychiatry 46: 681-688.
- Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R (1999) Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol Psychiatry 46: 56-72.
- Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, et al. (1997) Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci U S A 94: 2569-2574.
- Deutsch SI, Rosse RB, Schwartz BL, Mastropaolo J (2001) A revised excitotoxic hypothesis of schizophrenia: therapeutic implications. Clin Neuropharmacol 24: 43-49.
- Kawagoe KT, Garris PA, Wiedemann DJ, Wightman RM (1992) Regulation of transient dopamine concentration gradients in the microenvironment surrounding nerve terminals in the rat striatum. Neuroscience 51: 55-64.
- Schultz W (1998) Predictive reward signal of dopamineneurons. J Neurophysiol 80: 1-27.
- Hietala J, Syvälahti E, Vuorio K (1994) Striatal D2 receptor characteristics in neuroleptic-naive schizophrenic patients studied with positron emission tomography. Arch Gen Psychiatry 51: 116â��123.
- Reith J, Benkelfat C, Sherwin A, Yasuhara Y, Kuwabara H, et al. (1994) Elevated dopa decarboxylase activity in living brain of patients with psychosis. Proc Natl Acad Sci USA 91:11651-11654.
- Kumakura Y, Cumming P, Vernaleken I, Buchholz HG, Siessmeier T, et al. (2007) Elevated [18F] Fluorodopamine Turnover in Brain of Patients with Schizophrenia: An [18F] Fluorodopa/Positron Emission Tomography Study. J Neurosci 27: 8080-8087.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 18044
- [From(publication date):
July-2013 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 13375
- PDF downloads : 4669