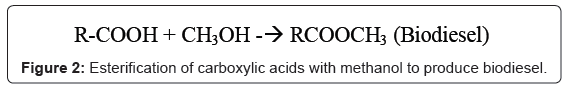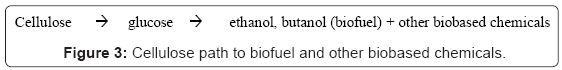Research Article Open Access
A Single Bio-based Catalyst for Bio-fuel and Bio-diesel
Jahangir Emrani1* and Abolghasem Shahbazi21Department of Chemistry, North Carolina A&T State University, Greensboro, NC 27411, USA
2Department of Biological Engineering, North Carolina A&T State University, Greensboro, NC 27411, USA
- Corresponding Author:
- Jahangir Emrani
Department of Chemistry
North Carolina A&T State University
Greensboro, NC 2741, USA
E-mail: emrani@ncat.edu
Received date: November 19, 2011; Accepted date: January 11, 2012; Published date: January 13, 2012
Citation: Emrani J, Shahbazi A (2012) A Single Bio-based Catalyst for Bio-fuel and Bio-diesel. J Biotechnol Biomaterial 2:124. doi:10.4172/2155-952X.1000124
Copyright: © 2012 Emrani J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
Current homogeneous catalysts used for commercial biodiesel synthesis are toxic and flammable. For Feedstock, their reaction requires refined oil which is a human food and expensive. In addition, due to contamination, the main byproduct of their reaction, glycerol is not usable for sale as a high value product and is land filled or burned as waste. Furthermore, the use of these catalysts in conversion of low cost feed-stocks such as waste oil and greases is not economical because their reactions produce soaps and clean up of the soap imposes additional processing cost to the biodiesel synthesis process. On the other hand, heterogeneous catalysts under development for biodiesel synthesis are either derived from non-renewable resources, or have problems with toxicity or stability. The solid heterogeneous bio-based catalysts are based on renewable resources, non-toxic, stable, effective, and low cost and are expected to work well for conversion of low cost feed-stocks such as waste oil and grease such as yellow, brown and black grease. In addition to catalyzing biodiesel synthesis, biobased catalysts can convert cellulosic agricultural waste to biofuel via saccarification followed by fermentation. Due to the abundance of cellulosic biomass in the nature, this reaction has great significance as it will affect the availability of not only biofuel from the fermentation of glucose, but also the availability of all organic chemicals and even hydrogen fuel from biomass. Because the catalytic active site in these catalysts are chemically bound, in contrast to other similar catalysts such as naphthalene sulfonic acid, the catalytic active sites of biobased catalysts will not break down, or leach. As a result, both the biodiesel and the glycerol by product will be free of catalyst contaminants. This allows the biodiesel to be safely burned and the glycerol byproduct to be sold as value-added commodity for pharmaceutical and cosmetic uses.
Keywords
Biobased catalyst; Biofuel: Biodiesel; Homogeneous catalyst; Heterogeneous catalyst; Cellulosic biomass; Fermentation; Naphthalene sulfonic acid; Free fatty acids; Trap grease; Cooking oil; Brown oil; Base catalyst; Environmentally friendly; Zeolites; Amberlyst-15; Nafion, sulfonation; Glucose; Cellulose; Saccarification; Bioethanol; Aromatic organic compounds; Polycyclic aromatic; Grapheme; Sulfonated biobased catalyst; Cyclodextrin; Sulfonated biochar; SO3H
Introduction
Development of a cost effective and environmentally friendly biodiesel synthesis has been the driving force behind recent research activities and interest in biobased catalysts. Such catalysts not only catalyze transesterification of triglycerides to form biodiesel (Figure 1), they also catalyze esterification of free fatty acids (FFA) to form biodiesel even in the presence of significant amount of water (Figure 2). Cooking oil, trap grease and brown oil are abundant low cost feedstock containing large percentage of FFA with potential to make the dream of affordable biodiesel a reality (Figure 2). But due to their nature, this feedstock’s collected from the restaurants and food processing facilities waste; contain a lot of water that prohibits their conversion to biodiesel using the conventional catalysts.
Conventional catalysts such as NaOH and KOH catalyze the reaction homogeneous reaction but the work up of the process requires a lot of water to wash the byproduct soap. This requirement makes these catalysts environmentally unfriendly. Biodiesel synthesis reaction using heterogeneous base catalysts including Ca ZrO3 , Al2O3-SnO, Li-MgO, Al2O3-KI, KOH-Al2O3, KOH-NaY, K2CO3- Al2O3 and silica require anhydrous refined oil as feedstock which contains less than 0.5% FFA. Refined oil with low FFA content is expensive. For this reason, using these catalysts to make biodiesel is not economical. The homogeneous acid catalyst H3SO4 is good for esterification and transesterification but it corrodes the equipments and its process requires a lot of water wash which makes it environmentally unfriendly.
Heterogeneous acid catalysts such as zeolites (La/zeolite beta), MCM-41, Amberlyst-15, Nafion, and niobic acids will catalyze both transesterification and esterfification reactions [1] and because they are heterogeneous, can easily be separated from the reaction mixture and recycled. They also produce no soap. However, because these catalysts catalyze the reaction via a Bronsted acid mechanism using OH group, their activity will go down over time in the presence of water.
In addition, they are expensive and have low number of acid sites. Enzymes as catalyst for these reactions are also too expensive at this time. However, there is always a possibility for their prices to come down to make their biodiesel synthesis process economical.
Biobased catalysts which can be made by sulfonation of cellulosic agricultural waste can efficiently catalyze these reactions in both economical and environmentally friendly way. In contrast to nonbiobased catalysts which are derived from non-renewable resources and their recovery exerts damage to the environment (Table 1), these catalysts are not harmful to environment. In addition, biobased catalysts are active, stable, non toxic, non-corrosive and easy to separate from the products and fully active upon recycling. Among the characteristics of biobased catalysts, the number of acidic sites has the most dramatic effect on the catalytic activity while surface area has much less significant effect on the catalytic activity [2].
| NaOCH3 | Biobased Catalyst | Other Heterogeneous catalysts |
| Toxic and flammable, based on non-renewable resource, environmentally unfriendly | Non-toxic, cheap, based on renewable biomass, environmentally friendly | Expensive, based on non-renewable resource, environmentally unfriendly |
| Feedstock is refined oil which is a human food and expensive | Can use waste oil and grease as feedstock | Biodiesel synthesis are either derived from non-renewable resources such as rare metals, or have problems with toxicity or stability |
| Main byproduct of the reaction, glycerol is not usable and is land filled or burned as waste | Glycerol by product is clean and can be sold as high value product | |
| Not useful for conversion of low cost feed-stocks such as waste oil and greases because their reactions produce soaps and clean up of the soap imposes additional processing cost | Especially suited for trap grease and waste oil containing water and fatty acids |
Table 1: Comparison of properties of sodium methoxide, biobased catalysts, and other heterogeneous catalysts.
A second major process that has even more potential dramatic influence on the development and availability of renewable biobased fuel also uses biobased catalyst, not for making biodiesel but for the hydrolysis of cellulose to glucose. Once cellulose has been hydrolyzed to glucose, it can undergo fermentation to produce bioethanol, biobutanol, or other alcohols which can be added to gasoline and used as transportation fuel (Figure 3). Alternatively, hydrogen and just about any organic compound may be obtained from the sugar directly or through transformations to replace petroleum derived organic chemicals.
The goal of this paper is to put the two catalytic functions of the biobased catalysts together and offer a single unique catalyst which not only can convert oil and waste oil to biodiesel, but also cellulose to bioethanol through saccarification/fermentation. Such special characteristics of the biobased catalysts allow low cost production of both bio-fuels and biodiesel in an environmentally friendly way with a positive impact on the job market. A biobased catalyst can also be effectively used to carry out other acid catalyzed organic reactions. Advantages of these catalysts stem from reduction of environmental pollution because they are made from waste, they are active, stable and they lower the biodiesel cost by using waste grease containing water and fatty acids.
The Sulfonation Reaction
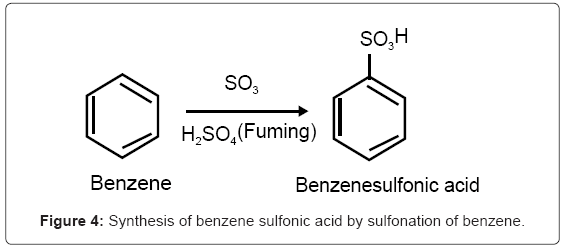
We discuss the sulfonation reaction because biobased catalysts are prepared by sulfonating cellulosic biomass. Sulfonated aromatic organic compounds are synthesized by direct treatment of the organic compounds with the low priced fuming sulfuric acid. The reaction of the acid with benzene which is exothermic produces bezenesulfonic acid (Figure 4).
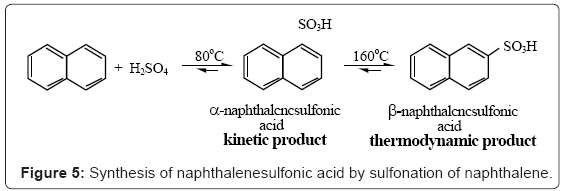
If we instead use naphthalene, alpha and beta naphthalene sulfonic acids will be obtained (Figure 5).
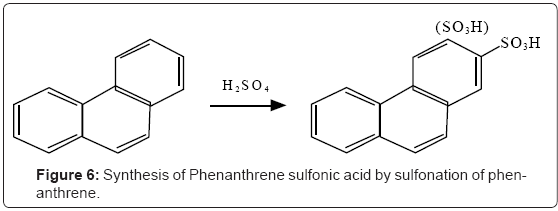
With phenanthrene, 2-, and 3-phenanthrenesulfonic acids are synthesized (Figure 6).
These small sulfonated aromatic compounds show excellent catalytic activity for biodiesel synthesis. However, they are not stable as catalysts because aromatic molecules leach out of them over time [3]. To prevent leaching, catalysts with larger molecular size are prepared starting from macromolecule carbohydrates.
I-Biobased catalyst for biodiesel synthesis
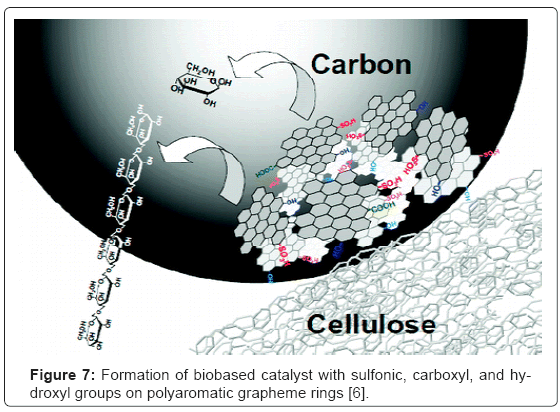
These catalysts are made by sulfonation of a partially carbonized carbohydrate. Partially carbonized carbohydrate may be prepared in the lab by following a procedure described below, or it may be purchased from commercial sources [4]. The structure of these catalysts has been shown to consist of grapheme like polycyclic hydrocarbons which bears sulfonate groups at the edges (Figure 7,8) [5]. Although it is the sulfonate groups that are the active catalyst sites, the rest of the functional groups in the molecules of these catalyst still carry hydroxyl and carboxyl groups which result during the preparation steps of the catalyst. It is these secondary groups on the catalyst which give the biobased catalyst their edge over other sulfonated and metal based heterogeneous acidic catalysts such as zeolites. In fact, it has been suggested that such a structure allows the biobased catalysts to behave as enzymes in taking a substrate such as fatty acid in their active site, catalyze its esterification and then release it into the reaction medium (Figure 7).
Below, we discuss the synthesis of biobased catalysts from glucose, sucrose, cellulose and other carbohydrates. We also discuss the reactions conditions for the preparation of each catalyst and their active sites characterization. We will also discuss the application of these catalysts in biodiesel synthesis.
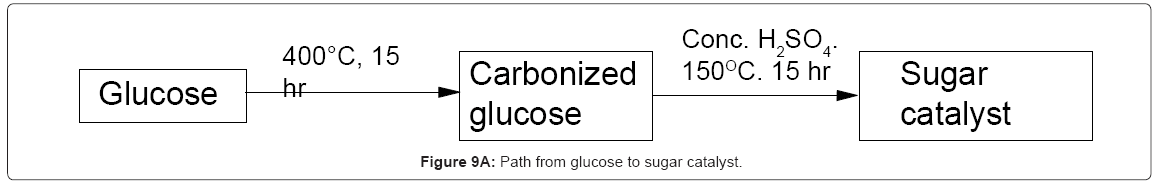
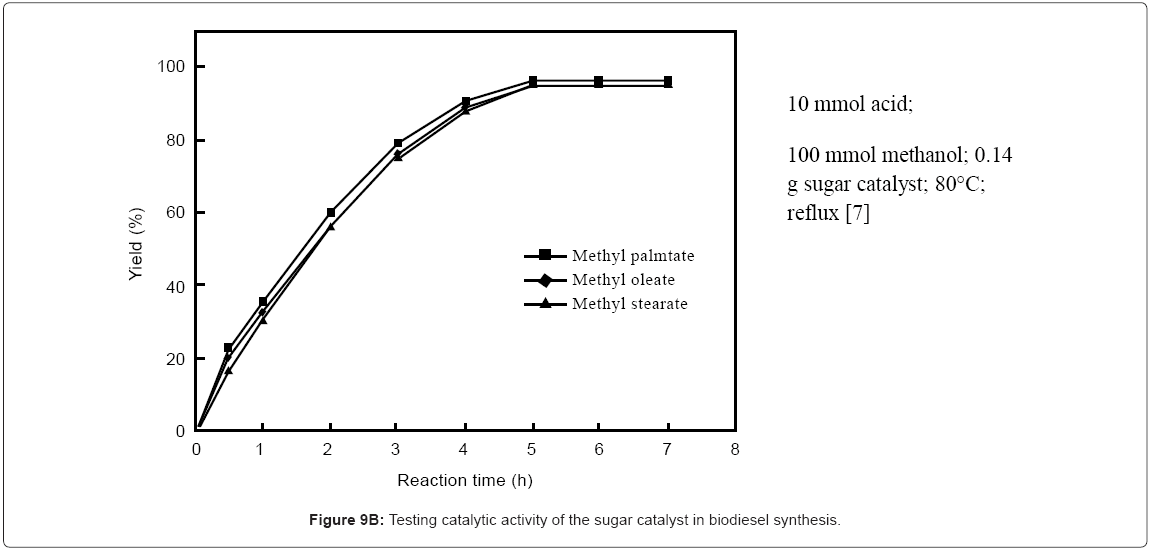
I-1.Biobased catalyst from glucose and sucrose: In 2005, Toda and coworkers et al. [5] reported the synthesis of a high performance solid catalyst from D-glucose and from sucrose by pyrolysis of the sugars at low temperature to induce partial carbonization to polycyclic aromatics as a black powder which was then sulfonated using concentrated sulfuric acid. The catalyst was insoluble in water, methanol, benzene, hexane, DMF and fatty acids (Figure 9A). The particles of the catalyst showed catalytic activity and stability even when bound together by a binding polymer (which incidentally also acted as proton conductor like Nafion). The biobased catalyst was more active than other solid acid catalysts but weaker than sulfuric acid and its activity was not lost in the reaction (Figure 9B). The activity was higher and more stable than naphthalene sulfonic acid [3]. Stability of the sugar based catalyst over multiple uses was confirmed [7]. Also, it was shows that the biobased catalyst had better performance than other solid acid catalysts [8]. Also, it was shown that pyrolysis temperature at which carbonized D-glucose is made has direct influence on the catalytic activity of the resulting catalyst. For example, above 723K, glucose is converted to a rigid material which after sulfonation does not show any catalytic activity. Values for D-glucose derived sulfonated catalyst have been summarized [2]. These data show that the sulfonic group has significant effect on the catalytic activity and as the number of sulfonic acids increase, so does the activity of the catalyst.
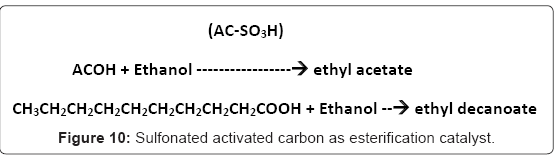
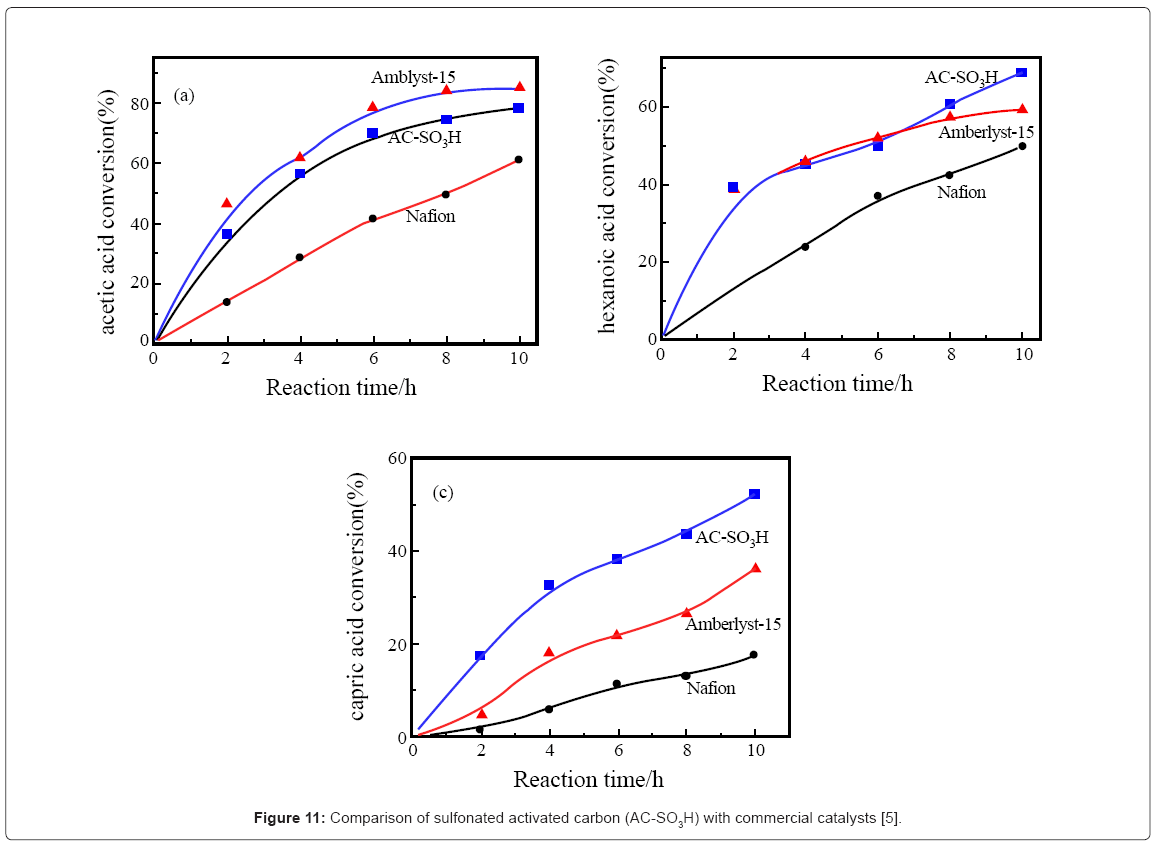
I-2.Biobased catalyst from starch and activated carbon (polycyclic aromatics): Toda et al. [5] who first made the biobased catalyst from D-glucose also synthesized biobased catalysts from cellulose and starch and reported on the activity of the resulting sulfonated activated carbon. In the esterification of acetic and decanoic acid with ethanol (Figure 10,11), activated carbon catalyst (AC-SO3H) showed similar activity as Nafion NR50 and Amberlyst-15, two commercial catalysts with similar functional groups. The activated carbon catalyst AC-SO3H with 0.64mmol/g SO3H and sp. area of 602m2/g gave 78% while the Amberlyst-15 with 4.60 mmol/g SO3H groups gave 86% yield. The yield was higher for the longer chain decanoic acid but the leaching of the activated carbon catalyst was a problem [9].
I-3.Biobased catalyst from cellulose and cyclodextrin: Using concentrated or fuming sulfuric acid, the biomass pyrolysis product, biochar was sulfonated. The resulting catalyst worked well for esterification of fatty acids but not so well for the transesterification of FFA in oils. In terms of mechanism, it was discovered that the catalyst with the highest surface area and acid density showed the highest activity for biodiesel production. Also, catalyst with the highest surface area was the best for transesterification. By increasing the alcohol to oil ratio, conversion of free acids (FFA) is increased. The same was true for reaction time and catalyst loading [2]. In addition to cellulose, cyclodextrin was also sulfonated to make the biobased catalyst [11]. Catalyst which was made from incompletely carbonized cyclodextrin was effective in the conversion of acetic and oleic acids to the corresponding methyl, ethyl, and butyl esters in 68-87% yield.
I-4.Biobased catalyst from agricultural waste: Sulfonated biochar obtained from the fast pyrolysis of woody biomass (a mixture of wood waste, white wood, bark, and shavings obtained from Dynamotive Energy Systems Corporation) was effective as biodiesel catalyst. The catalyst carbonized at 675°C showed the highest catalytic activity. It was discovered that the carbonization temperature has significant effect on the surface area, total acid density, and activity of the catalyst. The yield for the catalyzed reaction was 24.5-44.2% [2].
Carbonized vegetable ashphalt was also sulfonated to obtain a catalyst which worked well for the simultaneous esterification and transesterification reactions leading to biodiesel. At 220°C, within 4 hrs, conversions were 80.5% for the triglyceride and 94.8% for FFA. Properties of this catalyst were established in the study [12]. Also, among agricultural wastes, carbon-based catalyst from de-oiled canola meal was tested for the conversion of canola oil to biodiesel (Figure 12): Partially carbonized peanut shell also gave a good catalyst following sulfonation. The catalyst which had amorphous porous structure was characterized by SEM, EDS, BET, FTIR, NH3TPD, and TGA. It showed high activity and stability. It was effective in converting glycerol to glycerol ethers including mono, di- and tri-tert-butylglycerol [13].
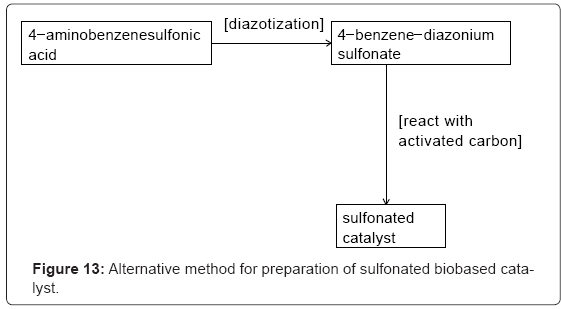
I-5.Other methods of making sulfonated (biobased) catalysts: Poly (styrene sulfonic acid)-grafted carbon nanotube was synthesized and used for alkylation of hydroquinone using tertiary-butanol. A conversion of 73.3% and yield of 53.7% was obtained at 150°C [14]. Also, polymer impregnated with glucose after pyrolysis and sulfonation afforded a catalyst with superior stability and activity due to its higher acid densities [15]. Sulfonated activated carbon was synthesized by reduction of aryl diazonium salt and its activity was compared with Nafion and Amberlyst-15, two commercial sulfonated catalysts (Figure 13) [9].
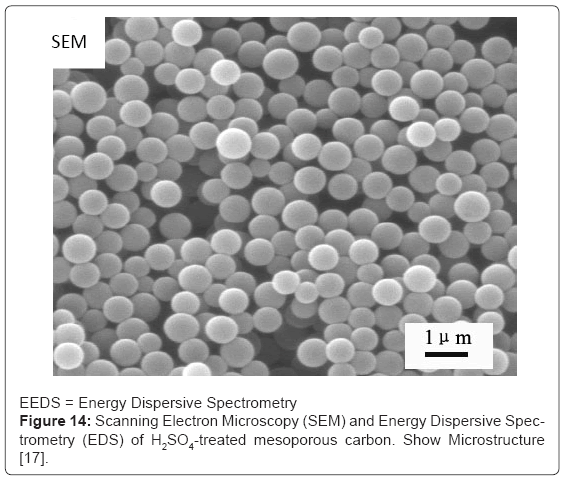
Better catalytic activity for making biodiesel type molecules, was found to be due to the large surface area and mesoporous structure of the activated carbon [9]. Sulfonation of FDU-15, a mesoporous polymer, produced a catalyst which affected 99% conversion in esterification of palmitic acid using methanol at 343k temperature. Using soybean oil, the transesterification reaction gave 99% yield at 413k and a methanol /oil ratio of 1:1 [16]. Mesoporous carbon and carbon nanotube have also been sulfonated using sulfuric acid to make the sulfonated catalysts (Figure 14) [17].
Mesoporous carbon--→ carbon based solid catalyst
Carbon nanotube ----→ carbon based solid catalyst
I-6.Stability of the biobased catalysts: Contrary to other workers [7], Mo et al. found that the sugar catalyst loses its activity over time due to leaching [Journal of Catalysis, 254, no2, 2008, 332-338 [7b]. This shows that depending on its preparation conditions, the sugar catalyst may be susceptible to leaching. With cellulose based catalyst, due to the larger size of the catalyst molecules, stability is expected to be better.
I-7.Biobased catalyst from sulfonated carbon mixed with silica or other elements: Sulfonated carbon catalyst in composition with iron was synthesized and characterized using SEM, TEM, XRD, FTIR, Vibrating sample magnetometry. The catalyst which showed supermagnetic properties hydrolyzed cellulose with 40.6% conversion but lost activity quickly [19].
Biobased catalyst containing silica has shown selectivity for specific organic reactions such as dimerization of alpha-methylstyrene [20]. Here, the biobased catalyst without the silica was not able to catalyze the reaction due to insufficient surface area.
Also, zinc impregnated wood powder was sulfonated to make sulfonated catalyst (Figure 15) [21].
When sucrose was impregnated with aluminum oxide and the then carbonized under nitrogen at 600°C, it gave a graphene layer on top of aluminum oxide which was separated by removing the aluminum layer using hydrogen fluoride [22].
Carbonization/sulfonation was repeated multiple times [22]. As the carbon content in the carbon coated alumina increased, catalysts dispersion became poor and lower yield resulted. High catalytic activity was related to good dispersion of the catalysts in methanol. Catalysts with improved dispersion showed the highest yield, seven times that of Amberlyst-15. The samples prepared at higher temperature had lower SO3H density. The samples carbonized at lower temp, had smaller carbon sheets, more SO3H, because SO3H attaches to the edge of the sheet. Pore size distribution and N2 sorption isotherm for the catalyst were also reported.
Biobased catalyst cannot be prepared by sulfonation of graphite, carbon black, graphitized carbon fiber, activated carbon, or glassy carbon.
I-8.Other biobased catalysts: Hydrothermal carbonization of furahdehyde and p-toluenesulfonic acid in water at 180°C gave the carbon based catalyst in 4hrs. The catalyst showed superior activity for esterification and oxathioketalization [23]. Same type of catalyst prepared by others showed great yield of 95% for oxathioacetalization. Catalyst was stable, active, low cost and reusable [24]. Another catalyst made the same way using a one step hydrothermal carbonization of glucose, citric acid, and hydroxyethylsulfonic acid at 180°C in 4 hours showed similar activity [25].
I-9.Procedure for preparation of biobased catalyst: In one case, carbonized biomass or biochar from commercial sources was used [2] as starting material for making the catalyst. In rest of the cases, carbohydrate was first carbonized and the carbonized biomass was ground up into a powder. The powder was then mixed with concentrated or fuming sulfuric acid at a ratio of 15:1 or similar, and heated under nitrogen at 150°C for 15hrs [5]. The black solid powder was washed thoroughly with distilled water till no acidity could be detected in the water wash. The resulting solid catalyst was dried at 110°C overnight.
I-10.Characterization of the acidic functional groups in the biobased catalyst: There are three acidic groups in the catalyst, the carboxyl and phenolic hydroxyl group which result from carbonization of biomass, and the sulfonate group which results from reaction of carbonized biomass with sulfuric acid. To determine the number of these acidic sites on the catalyst, a measured mass of the catalyst in the powder form in water is titrated using base such as sodium hydroxide and a titrator. In practice, the acidic groups may be first treated with NaOH and backtitrated using HCl. To determine the acidity due to sulfonic acid, elemental analysis is performed which gives the amount of sulfur. This assumes that all the sulfur in the catalyst is in the form of SO3H. To identify SO3H groups, IR spectroscopy is used which shows characteristic bands at 1712, 1717, 1715cm-1. Symmetric S=O peak at 1032cm-1. phenolic OH shows peak at 1215 while carboxylic acid at 1700cm-1. To characterize the polyaromatic structure, XRD patterns are examined and patterns for samples at different temperatures are compared.
II-Biobased catalyst from based biobased catalyst as catalyst for cellulose hydrolysis
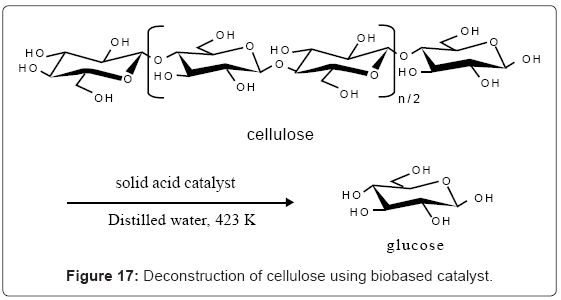
Breaking cellulose to produce glucose in an environmentally friendly fashion and at low cost is a major challenge for chemists and engineers today. This deconstruction reaction is however important in order for humans to be able to tap into the vast biobased resources which come in the form of cellulose. Once broken to glucose, glucose can be fermented to produce various liquid and gas fuels and just about all the commercially available organic chemicals (Figure 16). It turns out that amorphous carbon with SO3H, CO2H, and OH functional groups can hydrolyze crystalline cellulose which is 2nd focus of this paper. The activation energy for the process (110 kJ/mol) is less than the activation energy for reaction involving H2SO4(170 kJ/mol) as the catalyst. H2SO4 as catalyst can break down the cellulose but the challenge is the separation of the products from the aqueous mixture. A solid sulfonated catalyst can easily be separated by filtration.
Crystalline cellulose which is a glucose polymer cannot be hydrolyzed by dilute acids in crystalline form. Crystalline cellulose can be hydrolyzed by H2SO4but the process is expensive. Crystalline cellulose cannot also be hydrolyzed by strong solid acids such as niobic acid, H-mordenite, Nafion, amberlyte-15. To solubilize and deconstruct the cellulose, ionic liquids such as 1-butyl-3-methylimidazolium chloride with sulfonated carbon based catalyst have been tested. A 28% and 12.7% yield of reducing sugars was obtained in these reactions, respectively [25]. But with the low yield, this is not economical for large scale applications. Inorganic catalysts such as HNMoO6 [27] and H3PW12O40 has been used to catalyze the hydrolysis [26]. But due to the lower cost, non-toxic nature of the catalyst, and renewability, application of the biobased sugar catalyst if successful will be more advantageous. At 250°C, a sulfonated hyperbranched poly (arylene oxindole) catalyst was able to hydrolyze cellulose to levulinic acid [28]. Amorphous carbon bearing SO3H, COOH, and OH groups as catalyst gave 10% glucose yield at 373 K after 3 hr [10,29]. But, using the same type of catalyst, Onada obtained 40.5% yield of glucose at 423K in 24h [30]. Biobased catalysts in combination with silica or other supports have been able to break down cellulose. For example, sulfonated silica/ carbon nanocomposites hydrolyzed cellulose to glucose with high yield [31]. Catalyst prepared by sulfonation of partially carbonized cellulose contained graphene with 1.5 mmol/g SO3H, 0.4 mmol/g COOH, and 5.6 mmol/g phenolic OH groups. This catalyst showed high activity towards hydrolysis of cellobiose and crystalline cellulose (Figure 17). Niobic acid, and the commercial catalysts Nafion and amberlyst-15 were not able to hydrolyse these substrates [32]. Looking at these examples, it appears that high yield of glucose is achievable from breaking cellulose, but the right combination of catalyst, temperature, and solvent has to be found for consistent result at the large scale.
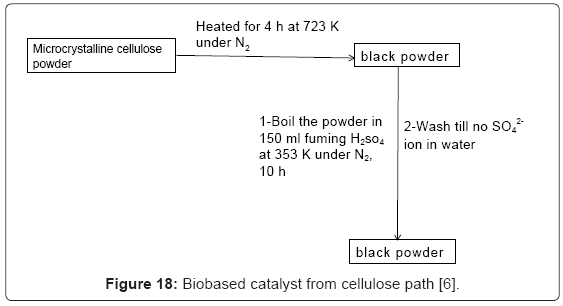
II-1.Procedure for deconstruction of cellulose [26]: Cellulose, 0.1 g, catalyst, 0.08 mmole, and distilled water, 5 ml were placed in a steel autoclave lined with Teflon. Autoclave was heated at 180°C for 2 hrs while sample was being agitated at 30rpm. Reaction mixture then cooled to 0°C in ice bath and filtered, weighed and the catalyst removed by filtration. The filtrate was analyzed by HPLC. Change of cellulose weight allowed calculation of % conversion. A higher than 90% conversions were observed and the catalyst showed high hydrothermal stability [30]. It was discovered that the amorphous carbon catalyst bearing SO3H, CO2H, and OH functional groups (Figure 18) was able to hydrolyze crystalline cellulose.
II-2.Hydrolysis of the cellulose reaction conditions: (Table 2).
| Container: | Pyrex reactor |
| Catalyst: | 0.300 g |
| Cellulose: | 0.025 g |
| Distilled water: | 0.700 g |
| Temperature: | 373K |
Table 2: Reaction mixture stirred (stir bar) in water or oil bath, analyze by LC, MALDI-TOF-Mass [6].
References
- West AH, Posarac D, Ellis N (2008) Assessment of four biodiesel production processes using HYSYS.Plant. Bioresour Technol 99: 6587-6601.
- Dehkhoda AM, West AH, NaokoE (2010) Biochar based solid acid catalyst for biodiesel production, Applied Catalysis A: General 382: 197-204.
- Hara M, Yoshida T, Takagaki A, Takata T, Kondo JN, et al. (2004) A carbonmaterial as a strong protonic acid. Angew Chem Int Ed Engl 43: 2955-2958.
- Mohan D, Pittman CU, Steelle PH (2006) Pyrolysis of Wood/Biomass for Bio-oil: A Critical Review. Energy Fuels 20: 848-889.
- Toda M, Takagak A, Okamura M, Kondo JN, Hayashi S, et al. (2005) Green chemistry - Biodiesel made with sugar catalyst. Nature 438: 178-178.
- Suganuma S, Nakajima K, Kitano M, Yamaguchi D, Kato H, et al. (2008) Hydrolysis of cellulose by amorphous carbon bearing SO3H, COOH, and OH groups. J Am Chem Soc 130: 12787-12793.
- Zong MH, Duan ZQ, Lou WY, Smith TJ, Wu H (2007) Preparation of a sugar catalyst and its use for highly efficient production of biodiesel. Green Chem 9: 434-437.
- Nakajima K, Hara K, Hayashi S (2007) Environmentally Benign Production of Chemicals and Energy Using a Carbon-Based Strong Solid Acid. J Amer Ceramic Soc 90: 3725-3734.
- Liu XY, Huang M, Ma HL, Zhang ZQ, Gao JM, et al. (2010) Preparation of a Carbon-Based Solid Acid Catalyst by Sulfonating Activated Carbon in a Chemical Reduction Process. Molecules 15: 7188-7196.
- Kitano M, Yamaguchi D, Suganuma S, Nakajima K, Kato H, et al. (2009) Adsorption-enhanced hydrolysis of b-1,4-Glucan on graphene-based amorphous carbon bearing SO3H, COOH, and OH groups. Langmuir 25: 5068-5075
- Shan C, Qian G (2011) Preparation of cyclodextrin-based carbonaceous catalyst and its application in the esterification. Journal of Wuhan University of Technology-Mater Sci Ed 26: 455-458.
- Shu Q, Gao J, Nawaz Z, Liao Y, Wang D , et al. (2010) Synthesis of biodiesel from waste vegetable oil with large amounts of free fatty acids using a carbon-based solid acid catalyst. J Applied Energy 87: 2589-2596.
- Zhao WQ (2010) Industrial & Engineering Chemistry Research 49: 12399-12404.
- Liua K, Lia C, Zhangb X, Huaa W, Yangb D, et al. (2010) Poly(styrene sulfonic acid)-grafted carbon nanotube as a stable protonic acid catalyst, Catalysis Communications 12: 217-221.
- Mo X, Looters E, Lu C, Liu Y, Goodwin JG (2008) A Novel Sulfonated Carbon Composite Solid Acid Catalyst for Biodiesel Synthesis. Catal Lett 123: 1-6.
- Lin Fang, Rong Xing, Hai Hong Wu, Xiao Hong Li, Yue Ming Liu, et al. (2005) Clean synthesis of biodiesel over solid acid catalysts of sulfonated mesopolymers, Science China Chemistry 53: 71481-1486.
- Peng F, Zhang L, Wang H, Lv P, Yu H (2005) Sulfonated carbon nanotubes as a strong protonic acid catalyst. Carbon 43: 2405-2408.
- Zong MH, Duan ZQ, Lou WY, Smith TJ, Wu H (2007) Preparation of a sugar catalyst and its use for highly efficient production of biodiesel. Green Chem 9: 434-437.
- Hua-yu W, Chang-bin Z, Hong H, Wang L (2010) Preparation o fMagnetic Sulfonated Carbon-Based Solid Acid Catalysts for the Hydrolysis, of Cellulose. Acta Phys Chim Sin 26: 1873-1878.
- Nakajima K, Okamura M, Kondo JN, Domen K, Tatsumi T, et al. (2009) Amorphous carbon bearing sulfonic acid groups in mesoporous silica as a selective catalyst. Chem Mater 21: 186-193.
- Kitano M, Arai K, Kodama A, Kousaka T, Nakajima K, et al. (2009) Preparation of a sulfonated porous carbon catalyst with high specific surface area. Catal Lett 131: 242-249.
- Genga L, Wanga Y, Yua G, Zhu Y (2011) Efficient carbon-based solid acid catalysts for the esterification of oleic acid. Catalysis Communications 13: 26-30.
- Xiao H, Guo Y, Liang X, Qi D (2012) One-step synthesis of a novel carbon-based strong acid catalyst through hydrothermal carbonization. Monatshefte für Chemie / Chemical Monthly 14: 8929-932.
- Lianga X, Xieb T, Qi C (2010) One-step synthesis of acid carbon and its catalytic activities for the oxathioacetalization. Solid State Sciences 12: 1270-1273.
- Rinaldi R, Palkovits R, Schuth F (2008) Depolymerization of cellulose Using Solid Catalysts in ionic Liquids. International Edition 47: 8047-8050.
- Tian J, Wang J, Zhao S, Jiang C, Zhang X, et al. (2010) Hydrolysis of cellulose by the heteropoly acid H3PW12O40. Cellulose 17: 587-594.
- Takagaki A, Tagusagawa V, Domen k (2008) Glucose production from saccharides using layered transition metal oxide and exfoliated nanosheets as a water-tolerant solid acid catalyst. Chem Commun 5363-5365.
- Vyver de SV, Thomas J, Geboers J, Keyzer S, Smet M, et al. (2011) Catalytic production of levulinic acid from cellulose and other biomass-derived carbohydrates with sulfonated hyperbranched poly (arylene oxindole)s. Energy Environ Sci 4: 3601-3610.
- Yamaguchi D, Kitano M, Suganuma S, Nakajima K, Kato H, et al. (2009) Hydrolysis of cellulose by a solid acid catalyst under optimal reaction conditions. J Phys Chem C 113: 3181-3188.
- Onda A, Ochi T, Yanagisawa K (2008) Selective hydrolysis of cellulose into glucose over solid acid catalysts. Green Chem 10: 1033-1037
- Vyver de SV, Peng L, Geboers J, Schepers H, Clippel de F, et al. (2010) Sulfonated silica/carbon nanocomposites as novel catalysts for hydrolysis of cellulose to glucose. Green Chem 12: 1560-1563.
- Suganumaa S, Nakajimaa K, Kitanob M, Yamaguchib D, Katoa H, et al. (2010) Synthesis and acid catalysis of cellulose-derived carbon-based solid acid. Solid State Sciences 12: 1029-1034.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 19250
- [From(publication date):
January-2012 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 14428
- PDF downloads : 4822