A Short Term Study on Toxic Effects of Distillery Sludge Amendment on Microbiological and Enzymatic Properties of Agricultural Soil in a Tropical City
Received: 09-Mar-2011 / Accepted Date: 20-Jul-2011 / Published Date: 25-Jul-2011 DOI: 10.4172/2157-7617.1000106
Abstract
Distillery sludge is an easy source of plant nutrient but its fertilizer value can vary considerably. In the present study anaerobically digested distillery sludge was applied to agricultural soils and its effect on soil biological and biochemical properties was evaluated. The sludge treatments were comprises of 0, 10, 50, 100 and 150 t ha-1 as single application in an agricultural field and tested for six months. Microbial respiration, microbial biomass carbon, FDA hydrolysis, phosphatase, urease and dehydrogenase activity were evaluated temporally throughout the incubation time for different amount of distillery sludge amendments. All biological parameters evaluated in this experiment were sensitive enough to show the effect of distillery sludge application on soil microorganisms. The results revealed that sludge application at different rates initially increased the microbial activity, its highest activity were found between 30 and 60 days after application when sludge was applied at the rate of 150 t ha-1 but after this the microbial activities decreases gradually. Results show that at applied dose and contact time soil microbial number increases but the diversity of soil microbial population decreases. Aerobic heterotrophic and symbiotic nitrogen fixing bacteria seems to be more sensitive to sludge addition and shows a marked decrease in population on higher doses. The results also shows that the sludge from distillery wastewater treatment plant may have potential as a beneficial soil amendment up to certain extent for improving biological properties of the soil but at higher doses its contamination can create harm for the beneficial soil inhabitant microbial population and their activities.
Keywords: Distillery sludge; Microbial biomass; Microbial respiration; FDA; Urease; Phosphatase; Dehydrogenase
5370Introduction
Distillery sludge application in degraded soils is amongst the most important economical resources for the soil fertility amelioration through improvement in soil water-holding capacity, texture, structure, nutrients retention, roots penetration, and reduction in soil acidity [1-3]. Presently due to the ever increasing number of sugar mills and distillery units in the country, it is now become mandatory to use the distillery sludge as a fertilizer to enhance the fertility levels of the soil. However, its application in soil also results in environmental problems [4] because apart from organic content and nutrients, sludge also includes toxic compounds, heavy metals, coloured compounds, dissolved inorganic salts, chlorinated lignin, and phenolic derivatives [5]. Thus, soil microorganisms may be affected via variation in soil temperature, pH, nutrient status, heavy metals, oxygen level and which in turn can affect the ecological processes linked with nutrients cycling [6]. Soil microbial activity, often linked with fertility levels, has been found to be indicated by microbial biomass, microbial respiration, hydrolysis of fluorescein diacetate (FDA), and activity of phosphatase and urease [7-9]. Microbial biomass is the 1-5% active fraction of organic matter of the soil, related with the soil fertility and is highly susceptible to soil pollution than the bulk organic matter. It acts as a reservoir of nutrients and gradually releases it into soil with passage of time which influences soil fertility levels. Soil respiration determines the effect of sludge amendment on soil microbial activity terms of carbon dioxide evolution. In this study, we have chosen enzymatic activities representative of main steps of soil biogeochemical nutrient cycles, i.e. C (FDA hydrolytic activity), N (urease), P (phosphatases), and microbial biomass (Dehydrogenase and FDA hydrolytic activity). Fluorescein diacetate has been used to determine amounts active microorganism. Hydrolysation product of FDA by a number of soil enzymes, such as proteases, lipases, and esterases is fluorescein, which can be easily visualized to estimate microbial activity. Urease catalyzes the hydrolysis of urea and amides to carbon dioxide and ammonia and helps in nitrogen and carbon cycling. Phosphatase is involved in phosphorus mineralization in soils through attack on phosphomonoesterase bond presents in soil organic matter thus increasing the phosphorus availability to microorganisms and plants. Dehydrogenase is a good indicator of metabolic activity soil microorganisms as it is not active independently of the parent microbial cell as extracellular enzymes in soil [10]. Short term evaluation of distillery effluent application in crop fields showed positive effect on crops growth [11-14] but long-term in-depth understanding of effect of distillery sludge is lacking on soil microbiological properties responsible for sustainability of soil. The present study, carried out in Indian tropical agro ecosystems, aims to evaluate the effect of varying amount of distillery sludge on soil microbiological characteristics including major soil microbial groups, microbial respiration, microbial biomass carbon and nitrogen, FDA hydrolysis, phosphate activity and some other soil fertility indices.
Materials and Methods
Experimental design, sampling and analysis
The study was performed at the agricultural farm at the Narang distillery at Nawabganj (26°52’ N, 82°08’ E and 98 m above the mean sea level). Experiment was designed in randomized block design with four replicate plots of 5 m×5 m size for various doses of sludge amendment. Likewise four un-amended plots were also established as control. The sludge was collected from sludge bed of ETP of distillery, sun dried in the form of cakes and placed in clean sterile polythene bags. Before application, distillery sludge was dehydrated and applied as single dose at a rate of 10, 50, 100 and 150 t ha-1 (on dry weight basis) in January 2009. Composite soil samples were collected from A-horizon (0–20 cm soil depth) of the plot without any crop at different time intervals from the experimental field. The experimental soil is an inceptisol with a pale brown colour, and sandy loam texture. The soil consisted of 9.5% clay, 10.5% silt, 16.5% coarse silt, 36.7% fine sand, 21.0% coarse sand, and 5.8% humic acids. Ten soil samples were collected and composited into one sample then packed in sterile polythene bags and were stored at 4°C in the dark and maintained at 50% water holding capacity then it was sieved through a 2mm-pore size sieve before use. The physico-chemical properties of sludge, amended and un-amended soil including organic matter, cation exchange capacity were estimated using standard methods [15,16]. Moisture content was determined by wet oxidation method. pH of sludge, un-amended and different sludge-amended soil (1:5 soil/sludge water suspensions) was determined by using digital pH meter. The electrical conductivity (EC) of was measured (1:5 soil/sludge water suspensions) with the help of conductivity meters. Samples were analyzed for total Nitrogen, Phosphorus and Potassium the extractable heavy metal concentrations in soil samples were measured by atomic absorption spectrometry after extraction with aquaregia.
Incubation and estimation of microbial respiration
Basal respiration was determined by the carbon dioxide released in the process of microbial respiration during 10 days of incubation after sludge application. Homogenized samples of fresh moist soil of 500 g (oven-dry basis at 105°C, 24 h), each for control and soil sludge mixtures, were placed into one l capacity jars, sealed properly and further incubated for at 25°C. The incubation of moist soil of each treatment in sealed jars was conducted for 180 days in a growth chamber at constant temperature (25±2°C); the humidity was adjusted to 50-60% of water holding capacity (WHC) and maintained at a constant level throughout the experiment by distilled water filled vial. Two 20 ml beakers were placed on top of the soil. One beaker contained 10 ml 0.5 M NaOH to absorb CO2 and the other contained 5 ml 2% H3BO3 to absorb NH3. The NaOH solution was changed after every 15 days. The H3BO3 solution contained phenolphthalein diluted into 100 mL ethanol (60%, v/v) as an indicator and was titrated once at the end of the incubation. Cumulated microbial respiration of the soils and soil-sludge mixtures were estimated by measuring CO2 evolution by alkali absorption of CO2 evolved periodically after each 15 days followed by titrating the residual NaOH with a solution of 0.1 M HCl after precipitating the carbonate with 1 mL of 1.5 M BaCl2 solution using phenolphthalein as indicator. The data were expressed as mg CO2-C 100 per gm of soil (Table 1) [17,18].
| Parameter | Sludge | Soil | 10 t ha-1 | 25 t ha-1 | 50 t ha-1 | 100 t ha-1 | 150 t ha-1 |
|---|---|---|---|---|---|---|---|
| pH | 9.2 ± 0.34 | 6.8 ± 0.27 | 7.3 ± 0.17 | 7.8 ± 0.38 | 8.47 ± 0.35 | 8.2 ± 0.31 | 8.2 ± 0.32 |
| CEC | 42.02 ± 1.56 | 22.64 ± 0.56 | 25.13 ± 0.82 | 28.35 ± 1.27 | 31.24 ± 2.69 | 38.67 ± 1.84 | 40.71 ± 1.56 |
| Moisture | 77 ± 2.12 | 70 ± 1.67 | 71 ± 1.62 | 72 ± 2.88 | 72 ± 3.43 | 74 ± 3.22 | 76 ± 3.71 |
| Organic matter | 25 ± 1.14 | 1.09 ± 0.03 | 3.09 ± 0.056 | 7.56 ± 0.31 | 13.26 ± 0.86 | 17.22 ± 0.75 | 19.09 ± 1.12 |
| EC (mScm-1) | 3.25 ± 0.13 | 1.55 ± 0.024 | 2.85 ± 0.12 | 2.87 ± 0.15 | 2.93 ± 0.083 | 3.02 ± 0.12 | 3.17 ± 0.17 |
| Phosphate | 313 ± 10.06 | 65 ± 2.88 | 76 ± 3.27 | 103 ± 4.82 | 183 ± 6.92 | 232 ± 8.43 | 264 ± 8.46 |
| Sulphate | 273.34 ± 8.45 | 55.68 ± 1.53 | 88.67 ± 1.82 | 120 ± 4.33 | 178.50 ± 6.45 | 211.36 ± 8.77 | 234.22 ± 9.39 |
| Phenol | 422.82 ± 7.21 | 3.09 ± 0.21 | 34.78 ± 1.47 | 89.12 ± 3.56 | 215.02 ± 8.82 | 322.45 ± 11.23 | 378.87 ± 12.56 |
| Total Nitrogen | 512 ± 11.56 | 86.76 ± 2.34 | 103.34 ± 2.66 | 126.56 ± 5.38 | 212.32 ± 7.33 | 371.56 ± 13.08 | 434.68 ± 13.33 |
| Amm. Nitrogen | 16.2 ± 0.68 | 19.5 ± 0.84 | 17.07 ± 0.63 | 17.32 ± 0.52 | 16.22 ± 0.73 | 17.34 ± 0.5 | 17.96 ± 0.72 |
| Sodium | 68.45 ± 3.56 | 23.67 ± 0.82 | 29 .14 ± 0.85 | 34.44 ± 1.72 | 48.45 ± 2.18 | 59.44 ± 2.07 | 62.34 ± 3.42 |
| Chloride | 245 ± 8.46 | 96.72 ± 1.51 | 109.28 ± 3.57 | 133.33 ± 4.07 | 168.45 ± 8.89 | 211.14 ± 9.19 | 221.34 ± 9.44 |
| Magnesium | 22.76 ±1.49 | 9.48 ± 0.32 | 9.88 ± 0.48 | 11.2 ± 0.43 | 14.42 ± 0.77 | 17.22 ± 0.63 | 18.86 ± 0.42 |
| Calcium | 76.22 ± 2.62 | 11.55 ± 0.21 | 19.33 ± 0.64 | 22.34 ± 0.83 | 45.3 ± 2.21 | 62.12 ± 3.46 | 69.25 ± 3.28 |
| Aluminum | 13.6 ± 0.62 | 3.94 ± 0.27 | 4.34 ± 0.47 | 5.12 ± 0.13 | 8.12 ± 0.34 | 10.88 ± 0.64 | 11.2 ± 0.82 |
| Potassium | 18.22 ± 0.81 | 0.39 ± 0.017 | 2.82 ± 0.13 | 5.44 ± 0.21 | 8.55 ± 0.41 | 13.33 ± 0.88 | 15.77 ± 0.75 |
| Cadmium | 1.89 ± 0.03 | 0.06 ± 0.0021 | 0.32 ± 0.016 | 0.74 ± 0.017 | 1.02 ± 0.042 | 1.38 ± 0.04 | 1.56 ± 0.037 |
| Chromium | 2.25 ± 0.08 | 0.89 ± 0.014 | 2.88 ± 0.065 | 2.89 ± 0.054 | 2.92 ± 0.12 | 3.08 ± 0.07 | 3.16 ± 0.076 |
| Copper | 165.02 ± 6.62 | 2.25 ± 0.043 | 18.22 ± 0.78 | 34.77 ± 1.05 | 62.88 ± 3.27 | 88.17 ± 4.77 | 104.12 ± 5.72 |
| Iron | 97.08 ± 4.38 | 3.73 ± 0.069 | 19.12 ± 0.67 | 31.12 ± 1.65 | 54.34 ± 2.11 | 73.74 ± 3.38 | 79.44 ± 4.41 |
| Manganese | 102.73 ± 2.28 | 1.86 ± 0.047 | 12.66 ± 0.48 | 17.44 ± 1.02 | 58.55 ± 1.94 | 62.55 ± 2.7 | 81.26 ± 3.88 |
| Nickel | 34.18 ± 1.23 | 4.11 ± 0.021 | 13.66 ± 0.63 | 17.59 ± 0.82 | 20.33 ± 0.87 | 25.6 ± 1.34 | 29.12 ± 1.23 |
| Zinc | 167.46 ± 6.18 | 12.18 ± 0.15 | 18.65 ± 0.86 | 21.34 ± 1.67 | 46.55 ± 2.24 | 51.61 ± 2.45 | 68.12 ± 2.88 |
| Lead | 22.18 ± 0.83 | 4.31 ± 0.062 | 8.86 ± 0.32 | 14.77 ± 0.73 | 23.72 ± 1.28 | 22.44 ± 1.55 | 22.32 ± 1.08 |
Table 1: Physicochemical properties of soil, distillery sludge, and sludge amended soil (values represent mean n=3 ±SE). All values presented in mg kg-1 except electrical conductivity (mScm-1) and pH.
Soil microbial biomass carbon
Chloroform fumigation-extraction method [19] for the estimation microbial biomass carbon in soil, is considered as a representative method to estimate the whole soil functional entity [20-22]. 25 g each of fresh moist soil and soil-sludge mixture were fumigated with alcohol-free CHCl3 for 24 h at 25°C in a closed vessel. The organic carbon content of fumigated and control soil and soil sludge mixture was extracted with 0.5 M K2SO4 and further estimated by treatment with a mixture of 0.5 M sodium dichromate and sulfuric acid. An extraction efficiency coefficient of 0.45 was used to convert organic C to MBC [23]. Microbial biomass C was calculated as E2/kEC, where EC is organic C extracted from fumigated soils - organic C extracted from non-fumigated soils and kEC 0.45 [24-26].
Number of culturable microorganisms
Microbial populations in both amended and unamended fresh soil samples of 1 g each (in triplicate) were enumerated following standard serial dilution plating technique on selective media using one-fourth strength Ringers solution [27] and expressed as CFU g-1 dry soil. Culturally viable bacteria, fungi and actinomycetes were counted on nutrient glucose agar, Martin’s Rose Bengal Agar (amended with 30 mg/l streptomycin sulphate), and Kenknight’s Agar (amended with 0.05 g/l cyclohexamide), respectively [28]. Plates inoculated with 0.1 ml soil suspension cultured for 4, 7 and 10 days for heterotrophic bacteria, fungi and actinomycetes, respectively, at 28°C [29]. Nitrogen fixing bacteria enumeration in soil was based on the most probable number technique, using a semi-solid nitrogen-free combined carbon medium [30]. Microbial growth associated with acetylene reduction (indicating nitrogen-fixing bacteria) was assessed with the help of gas chromatograph Varian 3800GC (Varian Analytical Instruments, USA).
Enzyme activity
FDA activity: FDA activity (representing soil organic matter decomposition) was measured by incubating soil and soil-sludge mixtures (both 1.5 g) with 9.9 ml sodium phosphate buffer [8]. Hydrolysis, started at addition of 0.1 ml FDA solution (1 mg ml-1), was stopped after 1 hour of incubation at 25°C by adding 10 ml of acetone and absorbance of supernatant filtrate (by Whatman No. 40 filter paper) was measured at 490 nm. The concentration of the released fluorescein was calculated by pre-drawn standard curve and expressed as μg FDA g-1 h-1.
Urease: 0.5 mL toluene and 12 mL phosphate buffer mixed with 3g sieved soil and soil sludge mixtures and incubated for 15 min at 37°C in a BOD incubator. After mixing with 3 ml of 10 % urea solution it was again incubated for 1 hr at 37°C and 15 mL of a KCl 2 M solution containing 5 mg phenyl mercury acetate were added. The sample was agitated for 5 min and then filtered through a Whatman no.1 filter paper. 0.2 g magnesium oxide previously heated at 500-600 8°C for 1 hr was added to 10 microlitres of the filtrate to remove carbonates. The filtrate was added to 5 mL boric acid solution, with methyl red and bromocresol green as indicators and then titrated with a H2SO4 0.001 M standard solutions. A blank was prepared for each treatment, and the same procedure utilized for the samples was adopted, with the only difference that the urea solution was added after the KCl and phenylmercury acetate solution. Urease activity was expressed as μg N-NH4 +g-1.produced in 1 h of incubation [31].
Phosphatase: 5 g of soil sample, 0.25 ml of toluene, 1ml of 10 mM p-nitrophenyl phosphate and 10 ml of distilled water were mixed and after 1hr incubation 5 ml of 0.5 M CaCl2 and 20 ml of 0.5 M NaOH were added. The content was filtered using Whatman No.42 filter paper and volume made up to 50 ml with distilled water. The colour intensity was read at 420 nm. The concentration of phosphatase was obtained from a standard graph and the enzyme activity was expressed as μmol PNF g-1 h-1 [32,33].
Dehydrogenase: A 20 g of soil sample was taken in a boiling tube. To this 1 ml of 3% 2, 3, 5-Tri phenyl tetrazolium chloride (TTC) was added. Then 1 ml of 1 % glucose and 2.5 ml of distilled water was added and incubated for24 hrs at 37°C. After that 10 ml of methanol were added and incubated for another 5 hrs. The content was filtered and the samples were washed thoroughly with methanol. The red colour developed was read at 485 nm. The concentration of dehydrogenase in the sample was obtained from the standard graph using triphenyl farmazane. Results were expressed in μg TPF g-1 day-1 [33,34].
Results
Soils and sludge characteristics
Physico-chemical analysis of sludge was done in triplicate sets of samples showed that all the collected samples have high pH, EC, BOD, COD, TSS, TDS, phenolics and metals (Cu, Cd ,Cr, Zn, Fe, Ni, Mn, Pb etc) as shown in. High concentrations of nitrogen, phenol, chloride and heavy metals were detected in distillery sludge versus the agricultural soil .The values of pH (9.2) and EC (3.25 mScm−1) were also higher in sludge than the soil where the pH and EC were 6.8 and 1.55 mScm−1respectively. High values of pH and EC in the sludge may be due to the presence of high concentrations of soluble salt. High concentrations of heavy metals and salts in sludge are due to the condensation process, which takes place during sugar manufacturing and alcohol production. The effect of distillery sludge treatment on some soil chemical properties is also shown in the same which shows that certain properties increase from the control soil after amendment.
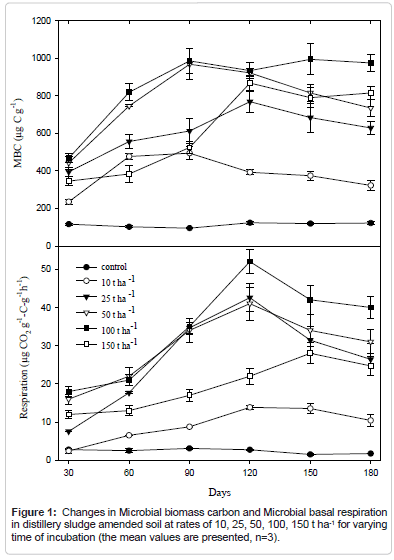
Respiration
The cumulative CO2 for control soil, sludge and for different soil sludge after 180 days of different soil sludge amendments was calculated. The microbial respiration of the sludge was many times higher than observed in soil. The addition of sludge increased the CO2 production of soils initially, increasing almost ten times the respiration activity when 150t ha-1 was applied, in respect to the unamended soils and then declined (Figure 1). This increment is associated with large amount of easily available soluble organic matter [3], nitrogen, phosphorous and other nutrient sources of the sludge that would have triggered microbial activity causing high rate of microbial-mineralization in the soil-mix [35]. The moistening of soil through addition of sludge plays a major role in increase in respiration which reactivates the growth and activity of microorganisms. The decline in CO, evolution in the later stage is caused by the exhaustion of one or more essential nutrients and the accumulation of toxic metabolites during incubation at higher doses of sludge application [36].
Microbial biomass C
Soil microbial biomass and biological activities is the indicator for changes in soil resulting from different stresses in soil ecosystems [37]. It was found from the experiment that the microbial biomass carbon in soil increased with the increment of sludge application and higher level (mg kg-1) was obtained after 90 days of incubation with the application of 150 t ha-1 of sludge (Figure 1). After 120 days of incubation, the MBC decreased in amended soil. It was seen that that application of water alone increases MBC. However, the values of MBC in amended soils were always higher than that of unamended control soil. All the incubation experiment is carried in controlled condition so that effect of seasonal change and other environmental factor in microbial biomass can be removed.
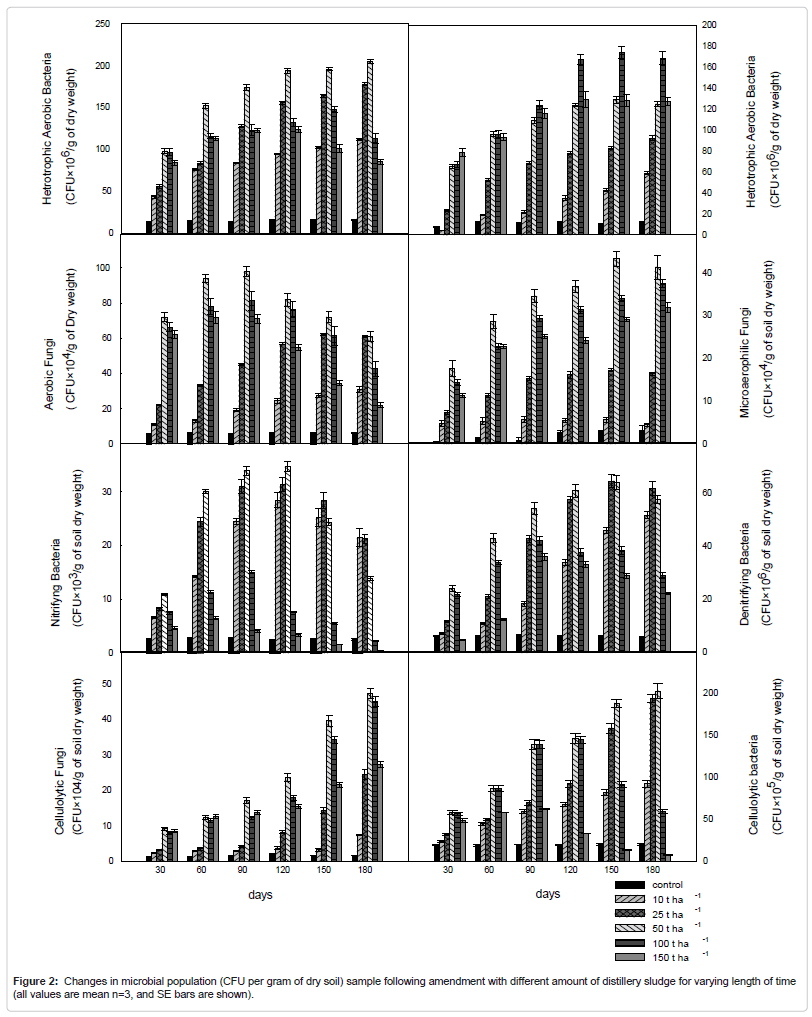
Microbial population
Quantitative analysis of soil microbial populations show variations in the populations of different groups of microflora in the soils following amendment with distillery sludge (Figure 2) The results show a marked decrease in total culture numbers of the different microbial groups for the highly contaminated soil samples. Aerobic heterotrophic, nitrifying and denitrifying bacteria seem to be more sensitive to high dose of distillery sludge contamination than the other microbial groups under evaluation. A difference in the viable counts of fungal and actinomycetes were also significant; however, these two microbial groups seemed to be less sensitive to the presence to the amendment.
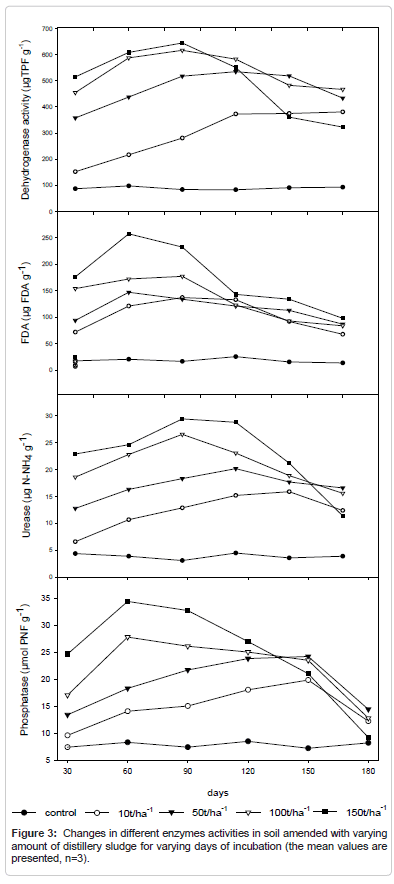
FDA activity
The FDA activity in soil increased with the increment of sludge application, and the higher level was obtained with the application of 150 t ha-1 of sludge, after 60 days of incubation (Figure 3). Results of FDA hydrolysis obtained with soil throughout the all incubation period (0-180 days), the FDA hydrolysis in sludge-amended soil was significantly different (P<0.05) compared to unamended. After 90 days of incubation, the FDA values decreased in both amended and unamended soil, however, the changes in values of FDA obtained in unamended soil was not very much significant. The increase of FDA hydrolysis indicates increment in the activity of the microorganisms due to organic matter contribution, macro and micronutrients, and pH increment by the sludge application. All these elements are essential for microbial growth and soil activity.
Urease activity
The mean urease activity was highest at the 150 t/ha application of distillery sludge i.e. while control shows the lowest activity (Figure 3). Enzyme activity changes both according to the level of application and incubation period during the experiment it was also seen that after application the soil show rapid increase in urease activity during incubation period when no additional amendment is applied. A slight decrease in urease activity is seen at 100 t/ha application of the sludge. Urease activity was expressed as mg N–NH+4 produced in 1 h of incubation.
Dehydrogenase activity
Dehydrogenase activity increased significantly at 150 tha-1 amendment on the 10th day of incubation (Figure 3). The activity increased within the period of incubation as reflected in the mean values within the days of incubation respectively. Dehydrogenase is an important enzyme for the oxidative processes in the soil which increases gradually with the rate of sludge application and its activity is very much dependent on the organic matter supply through distillery sludge. Our results are in agreement with [38,39] who also considered the activity of dehydrogenase, microbial biomass, soil respiration, and counts of Nfixing bacteria, etc. as sensitive bio-indicators of soil quality μgTPF/g.
Phosphatase activity
The phosphatase activity in distillery sludge treated soil is maximum when 150 t/ha sludge is applied and it further increases with the increase of the duration of incubation (Figure 3). It was maximum i.e. at the 180th day for the 150t/ha sludge application. Phosphatase recorded the maximum activity of 140.6 g kg hr that might have been favored by the copious quantity of phosphate present in the effluent. Sludge addition in experimental soil increases the plant-available phosphorus content and decreases acidity of the soils and consequently increases available P [40]. An increase of phosphorus availability in soil have been well correlated with the increase of phosphatase activity, being an adequate indicator of microbial activity and of modifications occurred in the soil due to sewage sludge application [9,33,41]. The increases the phosphatase activity is shown throughout incubation time (0-180 days), being significantly different (P<0.05) compared to unamended soils. The maximum activity was observed at 60 days of sludge application, and then the phosphatase activity decreased. experimental soil the levels of this enzymatic activity was 5 times higher after 60 days of incubation when 150 t ha-1 of sludge was applied, compared to unamended soil. The highest acid phosphatase activity observed in sludge-amended soils is similar to the results obtained for microbial biomass carbon and FDA hydrolysis, indicating that the sludge addition contributes to increase the overall microbial activity in this soil.
Discussion
It is observed that distillery sludge amendment influences physical, chemical and microbiological properties of the agricultural soil [8] which is in turn vital for the nutrient turnover and productivity [20,22,40]. Generally it is expected that the microbial activity in soil would always increase with the application of organic matter as distillery sludge, but, on higher doses negative effect by simultaneous enrichment of organic pollutants and heavy metal salts causes reduction in microbial biomass, activity and microbial populations of the soil. Experimental soils increased in organic matter, electrical conductivity, soluble heavy metals and toxic organic contaminants up to toxic level. The buffering capacity of the soil to degrade the pollutants is also not very effective at such a large quantity of toxic chemical addition [42]. The toxic effects of heavy metals and toxic organic substances present in distillery sludge on microbes are also determined by their solubility which is influenced its pH. This work showed that microbiological properties (MBC, microbial respiration, enzyme activity) increased as increasing rates of distillery sludge addition soils. Here the amount 50t ha-1 to100 t ha-1exhibited increasing tendency in terms of biological properties from 60 days to 120 days after application, thereafter it became lower at 180 days.
The quantitative analysis soil samples collected at different time period after different amount of application of distillery sludge showed continuous increasing counts of bacteria, fungi, and actinomycetes initially which were according to soil organic C and nutrient status when the concentration of the pollutants in the soil are below the toxic level then the number decreases drastically. At low doses it showed microbial population of reduced diversity but of greater number, biomass and activity, but at higher doses it causes decrease in number of microorganism, corresponding enzymatic activities and other microbiological properties. Aerobic heterotrophic and nitrogen fixing bacteria seem to be more sensitive to the toxic effects of distillery sludge than the other microbial groups under evaluation, undergoing a decrease in population size on higher doses. Differences in the viable count of fungal and actinomycetes were also significant; however, these two microbial groups seemed to be less sensitive to higher doses of sludge application in the soil.
The addition of sludge also increased the CO2 production and microbial biomass carbon of soils initially, increasing almost ten times when 150 tha-1 distillery sludge was applied, in respect to the unamended soils and then declined during the incubation period. Highest level was obtained after 90 days for MBC and 120 days for respiration of incubation with the application of both 150 t ha-1 and 100 t ha-1 of sludge then they decreased.
Enzyme activity changes both according to the level of application and incubation period during the experiment. The FDA hydrolysis activity in soil increased with the increment of sludge application, and the higher level was obtained with the application of 150 t ha-1 of sludge, after 60 days of incubation. After 90 days of incubation, the FDA values decreased, however, the changes in values of FDA obtained in unamended soil was not very much significant. Dehydrogenase activity increases up to 90th day of incubation. The activity increased many times within the period of incubation as reflected in the mean values. The phosphatase activity in distillery sludge treated soil is maximum when 150 t/ha sludge is applied but it further decreases with the increase of the duration of incubation. It was maximum i.e. at the 60th day for the 150t/ha sludge application. There was a rapid increase in urease activity initially in all the amendments and in the last phase of incubation there is a decrease in activity.
Conclusion
Distillery sludge amendment increased organic matter and electrical conductivity in the experimental soils and at high doses the amounts of soluble heavy metals and toxic organic contaminants were also higher up to toxic level. This work showed that microbiological properties (MBC, microbial respiration, enzyme activity) increased as increasing rates of distillery sludge addition in agricultural field soil in controlled conditions. Here the amount 50t ha-1 to 100 t ha-1 exhibited increasing tendency in terms of biological properties from 60 days to 120 days after application, thereafter it became lower at 180 days. 150 tha-1 distillery sludge shows negative effect on these properties comparatively very early. At the higher concentration of distillery sludge, decreasing tendency of soil fertility indicators observed becomes faster hence, we recommend the use of distillery sludge as improver of biological properties of soils if used in low concentration previously verifying its level of toxicity and stability to avoid potential hazardous effects over the soil microorganism. To minimize the shown environmental risks an adequate sludge management is proposed.
Acknowledgements
The authors are thankful to the University Grant Commission, New Delhi for their financial assistance and the Centre for Environmental Science & Technology, BHU, Varanasi, for technical help.
References
- O'Brien TA, Herbert SJ, Barker AV (2002) Growth of corn in varying mixtures of paper mill sludge and soil. Commun Soil Sci Plant anal 33: 635-646.
- Aravena C, Valentin C, Diez MC, Mora ML, Gallardo F (2007) Aplicación de lodos de planta de tratamiento de celulosa: efecto en algunas propiedades fÃsicas y quÃmicas de suelos volcánicos. J Soil Sci Plant Nutr 7: 1-14.
- Rato Nunes J, Cabral F, López-Piñeiro A (2008) Short-term effects on soil properties and wheat production from secondary paper sludge application on two Mediterranean agricultural soils. Bioresour Technol 99: 4935-4942.
- Cruz RL, Righetto AM, Nogueira MA (1991) Experimental investigation of soil groundwater impacts caused by vinasse disposal. Water Sci Tech 24: 77-85.
- Chandra R, Kumar P, Singh J (2004) Impact of an aerobically treated and untreated (raw) distillery effluent irrigation on soil micro flora, growth, total chlorophyll and protein contents of Phaseolus aureus. J Environ Biol 25: 381- 385.
- Chandra R, Yadav S, Mohan D (2008) Effect of distillery sludge on seed germination and growth parameters of green gram (Phaseolus mungo). J Hazard Mater 152: 431-439.
- Adam G, Duncan H (2001) Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol Biochem 33: 943-951.
- Sanchez-Monedero M, Mondini C, Cayuela M, Roig A, Contin M et al. (2008) Fluorescein diacetate hydrolysis, respiration and microbial biomass in freshly amended soils. Biol Fertil Soils 44: 885-890.
- Ros M, Pascual JA, Garcia C, Hernandez M, Insam H (2006) Hydrolase activities, microbial biomass and bacterial community in a soil after long term amendment with different composts. Soil Biol Biochem 38: 3443-3452.
- Quilchano C, Marañón T (2002) Dehydrogenase activity in Mediterranean forest soils. Biol Fertil Soils 35: 102-107.
- Bharagava RN, Chandra R, Rai V (2008) Phytoextraction of trace elements and physiological changes in Indian mustard plants (Brassica nigra L.) grown in post methanated distillery effluent (PMDE) irrigated soil. Bioresour Technol 99: 8316-8324.
- Joshi HC, Kalra N, Chaudhary A, Deb DL (1994) Asia Pacific J Environ Development 1: 92.
- Zalawadia NM, Patil RG, Raman S (1996) Effect of distillery waste water with fertilizer on onion and soil properties. J Indian Soc Soil Sci 44: 802.
- Ramana S, Biswas AK, Singh AB, Yadava RBR (2002) Relative efficacy of different distillery effluents on growth, nitrogen fixation and yield of groundnut. Bioresour Technol 81: 117-121.
- Kalra YP, Maynard DG (1991) Methods manual for forest soil and plant analysis. Information Report NOR-X-319 Forestry Canada, Northwest region, Northern Forest Centre, Edmonton, Alberta.
- APHA (2005) Standard methods for the examination of water and waste water. 21st Edn. American Public Health Association, Washington (D.C.).
- Hu S, Bruggen AHC (1997) Microbial dynamics associated with multiphasic decomposition of 14C-labeled cellulose in soil. Microb Ecol 33: 134-143.
- Alef K (1995) Methods in Applied Soil Microbiology and Biochemistry. New York: Academic Press 214-222.
- Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass. Soil Biol Biochem 19: 703-707.
- Goyal S, Chander K, Mundra MC, Kapoor KK (1999) Influence of inorganic fertilizers and organic amendments on soil organic matter and soil microbial properties under tropical conditions. Biol Fertil Soils 29: 196-200.
- Voroney RP, Winter JP, Beyaert RP (1993) Soil microbial biomass C and N In: Carter MR (ed) Soil sampling and methods of analysis. Canadian Society of Soil Science, Lewis, Chelsea 277-286.
- Hojati S, Nourbakhsh F (2006) Enzyme activities and microbial biomass carbon in a soil amended with organic and inorganic fertilizer. J Agron 5: 563-579.
- Vance ED, Brookes PC, Jenkinson DS (1987) Microbial biomass measurements in forest soils: the use of chloroform fumigation-incubation method in strongly acid soils. Soil Biology and Biochemistry 19: 697-702.
- Wu J, Joergensen RG, Pommerening B, Chaussod R, Brookes PC (1990) Measurement of soil microbial biomass C by fumigation-extraction. Soil Biol Biochem 22: 1167-1169.
- Joergensen RG (1995) The fumigation extraction method to estimate soil microbial biomass: Extraction with 0.01 M CaCl2. Agribiol Res 48: 319-324.
- He ZL, Yao HY, Chen GC, Huang CY (1997) Relationship of crop yield to microbial biomass in highly weathered soils of China. In: Ando T (Ed), Plant Nutrition for Sustainable Food Production and Environment. Kluwer Academic, Tokyo 751-752.
- Jensen V (1968) The plate count method In: Gray TRG, Parkinson D. The ecology of soil bacteria: An international symposium. Liverpool University Press, Liverpool 158-170.
- Allen ON (1953) Experiments in soil bacteriology. Burgess Publishing Company, Minneapolis.
- Oliveir A, Pampulha ME (2006) Effects of Long-Term Heavy Metal Contamination on Soil Microbial Characteristics. Journal of Bioscience and Bioengineering, the Society for Biotechnology, Japan 102: 157-161.
- Rennie RJ (1981) A single medium for the isolation of acetylene-reducing (dinitrogen-fixing) bacteria from soils. Can J Microbiol 27: 8-14.
- May PB, Douglas LA (1976) Assay for soil urease activity. Plant Soil 45: 301- 305.
- Halstead RL (1964) Phosphatase activity of soils as influenced by lime and other treatments. Can J Soil Sci 44: 137-144.
- Deng S, Tabatabai M (1997) Effect of tillage and residue management on enzyme activities in soils: III. Phosphatases and arylsulfatase. Biol Fertil Soils 24: 141-146.
- Casida LE, Klein DA, Santoro T (1964) Soil application dehydrogenase activity. Soil Sci 98: 371-376.
- Gagnon B, Lalande R, Fahmy F (2001) Organic matter and aggregation in a degraded potato soil as affected by raw and composted pulp residue. Biol Fertil Soils 34: 441-447.
- Wong JWC, Lai KM (1996) Effect of an artificial soil mix from coal fly ash and sewage sludge on soil microbial activity. Biol Fertil Soils 23: 420-424.
- Carter MR (1986) Microbial biomass as an index for tillage-induced changes in soil biological properties. Soil Tillage Res 7: 29-34.
- Tesarová M (2000) Kvalita pud a jejà biologické parametry. In: Pedologické dny. Kostelec nad Cernými lesy 85-91.
- Kubát J, Nováková J, Mikanová O, Simon T (1999) Selection of microbial methods for the bioindication of soil pollution. Pathways and consequences of the dissemination of pollutants in the biosphere II. Symp Praha 61-75.
- Gallardo F, Briceño G, Saravia C, Flores MJ, Sanhueza S, et al. (2009) Biological activity in a soil amended with pull mill sludge: A field study. III International Conference on Environmental, Industrial and Applied Microbiology. Bio.Micro. World , 2-4 December, Lisboa, Portugal.
- Fernandes SA, Bettiol W, Cerri C (2005) Effect of sewage sludge on microbial biomass, basal respiration, metabolic quotient and soil enzymatic activity. Appl Soil Ecol 30: 65-77.
- Nutter WL, Red JT (1985) Treatment of wastewater by application to forest land. Tech Assoc Pulp Pap Ind J 68: 114-117.
Citation: Tripathi BD (2011) A Short Term Study on Toxic Effects of Distillery Sludge Amendment on Microbiological and Enzymatic Properties of Agricultural Soil in a Tropical City. J Earth Sci Climat Change 1: 106. DOI: 10.4172/2157-7617.1000106
Copyright: ©2011 Tripathi BD. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 15437
- [From(publication date): 7-2011 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 10739
- PDF downloads: 4698