Research Article Open Access
A Novel Subspecies of Staphylococcus aureus from Sediments of Lanzhou Reach of the Yellow River Aerobically Reduces Hexavalent Chromium
| Xiaowei Zhang1, Lee R. Krumholz2, Zhengsheng Yu1, Yong Chen1, Pu Liu1 and Xiangkai Li1* | |
| 1MOE Key Laboratory of Cell Activities and Stress Adaptations, School of Life Sciences, Lanzhou University, Lanzhou, Gansu, 730000, P. R. China | |
| 2Department of Botany and Microbiology and Institute for Energy and the Environment, University of Oklahoma, Norman, Oklahoma, 73019, U.S.A | |
| Corresponding Author : | Xiangkai Li MOE Key Laboratory of Cell Activities and Stress Adaptations School of Life Sciences, Lanzhou University Lanzhou, P. R. China Tel: 86-931-8912561 Fax: 86-931-8912560 E-mail: xkli@lzu.edu.cn |
| Received March 04, 2013; Accepted May 23, 2013; Published May 25, 2013 | |
| Citation: Zhang X, Krumholz LR, Yu Z, Chen Y, Liu P, et al. (2013) A Novel Subspecies of Staphylococcus aureus from Sediments of Lanzhou Reach of the Yellow River Aerobically Reduces Hexavalent Chromium. J Bioremed Biodeg 4:188. doi: 10.4172/2155-6199.1000188 | |
| Copyright: © 2013 Zhang X, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Background: Lanzhou reach of the Yellow River is contaminated by heavy metals including chromium perennially. The microbial community within the sediment is very active. Yet the study on the bacteria in this distinctive microbial community is still scarce. Results: LZ-01, a Gram-positive hexavalent chromium-reducing bacterium was isolated from the soil sample collected at a petrochemical corporation wastewater discharge site of Lanzhou reach. It was able to aerobically reduce 94.5% of 0.4 mM Cr (VI) to Cr(III) in 120 hours. Cd (II) and NaN3 treatment both repressed Cr(VI) reduction in LZ-01 and Cr(III) precipitates were detected both on the cell membrane and in the cytoplasm by Transmission Electron Microscopy (TEM) imaging. LZ-01 also demonstrated resistance to 4 mM As (V) and 9 mM U (VI). LZ-01 was closely related to Staphylococcus aureus revealed by 16S rRNA sequence analysis. Comparison of cellular fatty acid components and Vitek phenotype identification provided further evidences that LZ-01 is a novel subspecies of S. aureus. Conclusions: A chromate-reducing bacterium LZ-01 identified as a novel subspecies of S. aureus was isolated from Lanzhou reach of the Yellow River. Cd (II) and NaN3 treatment and TEM images suggested that Cr(VI) was reduced not only intracellularly but also on the cell membrane. All the results indicate that the isolate has a great potential for bioremediation of Cr (VI)-contaminated environment.
| Keywords |
| Cr (VI) reduction; Bacterial isolation and identification; Staphylococcus aureus; Bioremediation |
| Introduction |
| Lanzhou city, located at the geometric center of China, is the largest city with a massive industrial base on the Yellow River. The Yellow River provides water for industrial, agricultural and domestic consumption in Lanzhou. Lanzhou reach of the Yellow River is about 50 km long. The upstream region is largely industrial while the downstream region is residential with a population of about four million [1]. Effluents containing high levels of metals and hydrocarbons from manufacturing facilities are continuously discharged to the river [2]. A previous study has shown that the metal concentrations (in μg/g dry soil sample) in the river bank soil is 13.68-48.11 (As), 26.39-77.66 (Pb), 89.80-201.88 (Zn), 41.49-128.30 (Cr), 29.72-102.22(Cu), and 773.23-1459.69 (Mn) [3]. Metal contamination of Lanzhou reach has caused widespread concern in China. So far researches on the environmental governance of Lanzhou reach have been limited to physics and chemical analyses [2,3] and investigation of microbial activity in the river bank soil is less common. |
| Chromium, an important industrial metal is one of the most harmful contaminants in Lanzhou reach due to the presence of industries of textile dyeing and painting, electroplating, plastic processing, all of which have led to Cr (VI) being discharged into natural ecosystems. Like most heavy metals, chromium has toxic effects on both humans and environmental organisms [4]. Trace amount of chromium is required for a variety of enzymes [5-7], while accumulation in the human body can cause cancer, growth and developmental abnormalities, neuromuscular control defects, renal malfunction, mental retardation and other illnesses [8]. Though chromium exists in varied valance states ranging from Cr(II) to Cr(VI), Cr(III) and Cr(VI) are the most stable forms in nature [9]. Trivalent chromium is insoluble with low mobility, while the hexavalent chromium species is soluble and highly mobile [10]. Previous studies revealed that Cr(VI) is almost 1000-fold more mutagenic and cytotoxic than Cr(III) [10,11]. Recent work has shown that the chromium concentration within the reach is about 8-25 times higher than the China water safety standard with the majority in hexavalent form [3]. Hence, control of Cr(VI) becomes an essential step of environmental remediation of Lanzhou reach. |
| A number of methods have been applied to reduce metal concentrations in soil. Physicochemical method are practiced and biological remediations are also taken into consideration [12-15]. Compared with physicochemical method, microbial remediation is more cost-effective and rarely introduces secondary pollution [16-19], especially for metal reduction in bioremediation. A wide variety of microorganisms capable of reducing aqueous hexavalent chromium ions have been reported including Staphylococcus epidermidis L-02 [11], Acinetobacter [20], Arthrobacter [21], Bacillus sp. [22], Desulfovibrio vulgaris [23], Pseudomonas sp. [24], Serratia marcescens [25], and Ochrobactrum sp. [26]. It is likely that microbes in Lanzhou reach river bank soil possess the ability to reduce Cr (VI) to Cr(III). Screening for these bacteria will be the first stage for microbial remediation of Cr(VI) in Lanzhou reach. |
| Staphylococcus aureus is a facultative Gram-positive coccus-shaped bacterium [27] that is best known for its ability to cause a range of illnesses [27,28]. Several recent studies report that S. aureus is involved in environmental metal remediation [4,29] and S. epidermidis which is closely related to S. aureus is able to anaerobically reduce Cr(VI) to Cr (III) [11]. However, there is no direct evidence that S. aureus can reduce Cr(VI). In this study, we screened the Lanzhou reach river bank soil for Cr(VI) reducers and isolated a novel subspecies of S. aureus strain LZ-01, which reduces Cr(VI) to Cr(III) aerobically. 16S rRNA sequencing and fatty lipids profiling showed that strain LZ-01 is closely related to S. aureus. Comparison of Vitek phenotype identification showed that there were 7 different reactions out of 64 between LZ-01 and type strains of Staphylococcus aureus. LZ-01 reduces Cr(VI) in a highly efficient manner suggesting that it may be useful for Cr(VI) reduction studies in Lanzhou reach. |
| Materials and Methods |
| Soil sample collection |
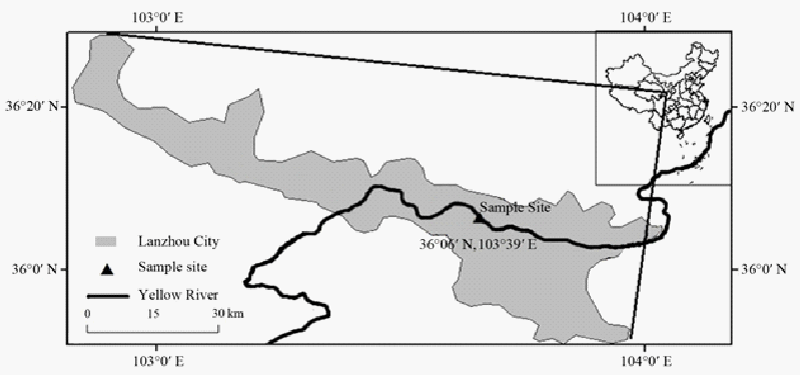
| The sampling site was 60 kilometers west of central Lanzhou city and adjacent to the upper reach of the Yellow River. The actual location was close to a petrochemical corporation waste water discharge site. The latitude and longitude of the sampling site is 36°06’N 103°39’E, (Figure 1). The pH of soils at the sampling site ranged from 5.0 to 6.0 with temperature around 18°C at the time of collection. The soil samples were collected from 15 cm below surface horizon and stored in sterile aluminum boxes at -20°C. |
| Screening for Cr(VI) -resistant bacteria |
| M9 minimal salts medium [30] used for isolations contained: 17 mM Na2HPO4, 22 mM KH2PO4, 19 mM NH4Cl and 0.1% yeast extract (w/v). The pH was adjusted to 7.4 prior to autoclaving and 2 mM sterilized MgSO4 and 0.2% (w/v) glucose were added after autoclaving. Solid M9 medium was prepared as above with 1.5% (w/v) agar. Filter sterilized solution of 1 M Cr(VI) was prepared from K2Cr2O7. Soil suspension was prepared by diluting 1 g fresh soil sample into 9 ml 0.85 mM NaCl solution and standing at room temperature for about 4 hours. For the isolation of Cr(VI)-resistant bacteria, 100 μl of soil suspension was added onto M9 solid medium with 2 mM Cr(VI). The plates were incubated at 37°C for 12-16 hours and 13 colonies were selected and then transferred into M9 liquid medium containing 2 mM Cr(VI). The liquid cultures were incubated on a shaker (180 rpm) at 37°C for 24 hours and then streaked onto solid M9 medium with 2 mM Cr(VI) and single colonies were picked after 24 hours incubation at 37°C. |
| Cr(VI) reduction assay of isolated strains |
| The isolated strains were cultivated at 37°C for 60 hours in M9 medium with Cr(VI) (0.4 mM, 1 mM, 2.5 mM, 5 mM, 7.5 mM and 9 mM). The initial and the final Cr(VI) concentration were determined according to the method reported previously [31]. Colony-Forming Units (CFU) and Cr(VI) reduction rates were compared between 6 treatments. |
| The selected strains were grown in M9 minimal salts medium with 0.4 mM Cr(VI) at 37°C for 120 hours. Liquid cultures were serially diluted to 10-5 every three hours to determine CFU by plating on M9 medium. The strains grown in M9 medium without Cr(VI) was used as control. At each time point, 0.5 ml liquid culture was removed and centrifuged at 12000 rpm for 5 min and the Cr(VI) concentration in the supernatant was measured. |
| The effect of biosorption and abiotic reduction was analyzed with Escherichia coli strain DH5α as control. Cells were grown with 0.4 mM Cr(VI) and after 120 hours the culture was centrifuged and Cr(VI) concentration in the supernatant was measured. The cell pellets were resuspended and treated with lysozyme at 37°C for 30 min and at 95°C for 15 min , and subjected to Cr(VI) measurement to determine sorbed Cr(VI) levels. The presence of Cr(III) in the cells was determined by first diluting 5 ml cell resuspension with 75 ml H2O, 10 ml H2SO4 solution (VH2SO4:VH2O=1:1), 10 ml 1% (w/v) AgNO3 solution and 2 g (NH4)2S2O8. Then the mixture was boiled for 2 min and subjected to Cr(VI) measurement. The concentration of Cr(III) within cells was calculated by subtracting Cr(VI) concentration in the cell resuspension before thiosulfate treatment from that after thiosulfate treatment. Cultures that were not inoculated were set up as controls. |
| Washed cell experiments were also performed with 50 ml overnight culture. Then cells harvested from 50 ml culture were added to 5 ml M9 medium with 0.4 mM Cr(VI). After 5 hours at 37°C, the dissolved and sorbed Cr(VI) and Cr(III) concentrations were determined as described above. |
| To investigate the mechanism of Cr(VI) reduction, we investigated the effects of inhibitors NaN3 and Cd(II). Since NaN3 is an inhibitor of cytochrome c3 which is a transmembrane protein and an essential component of the respiratory electron transport chain, it can repress the extracellular pathway [30]. And Cd(II) inhibits reactions involving sulfhydryl groups. It can inhibit thioredoxin which is an electron donor for cytoplasmic metal reductase [32]. The effect of NaN3 (3 mM) or Cd(II) (50 μM) on Cr(VI) reduction in washed cell experiments was determined by adding one inhibitor to M9 medium with 0.4 mM Cr(VI). After inoculation cells were incubated at 37°C for 12-16 hours and Cr(VI) concentrations were determined in culture supernatants. CFUs were measured after diluting the liquid culture to 10-5 in NaCl buffer (0.1% w/v). Cell suspensions were serially diluted to 10-5, and 100 μl of each dilution was then spread onto a M9 plate. Plates were removed from incubator after 2 to 3 days of incubation, and those ones with 50 to 500 colonies were counted. Controls were incubated with Cr(VI) only and inhibitors only. The concentration of Cr(VI) before and after incubation were also measured. All the growth experiments and treatments were conducted in triplicates and error bars presented in the figures were Standard Error of Mean (SEM) of triplicates. |
| Determination of pH and temperature optimum for Cr(VI) reduction |
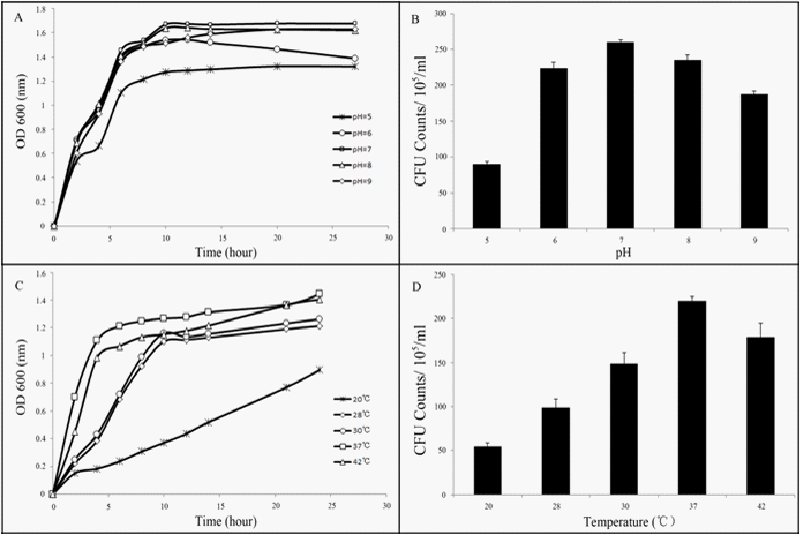
| Isolated strains were grown in M9 medium with 2 mM Cr(VI) at a range of pH values (5, 6, 7, 8, 9) at 180 rpm and 37°C. The temperature optimum was determined between 20°C and 42°C (20°C, 28°C, 30°C, 37°C, 42°C) at pH 7.4. All cultures were shaken at 180 rpm. Growth curves were measured by OD 600 and CFUs were counted after 12 hours incubation. |
| Determination of minimum inhibitory concentration (MIC) of As(V), Hg(II), U(VI) and Cr(VI) |
| Minimal Inhibitory Concentration (MIC) of each heavy metal was determined. The stock solution of Cr(VI), As(V), Hg(II), and U(VI) were prepared (0.5 M K2Cr2O7, 0.5 M 3 AsO4, 0.1 M HgCl2 and 0.1 M UO2(OAc)2·2H2O) in deionized water and autoclaved at 120°C for 20 min. To determine the MIC of Cr(VI), As(V) and Hg(II), M9 was used as base medium. To determine MIC of U(VI), a medium which contained 60 mM NaHCO3, 20 mM NH4Cl, 0.15 mM KH2PO4, 0.2 mM MgSO4, 0.1% (w/v) yeast extract, 0.2% (w/v) glucose was considered as base medium. All strains were incubated at 37°C, 180 rpm for 12-16 hours to obtain log phase culture. |
| Determination of antibiotic resistance |
| Concentration gradients of different antibiotics were set up in M9 medium as follows: ampicillin (0-10 mg/ml), chloramphenicol (0-2 mg/ml), vancomycin (0-1 mg/ml), kanamycin (0-300 μg/ml), erythromycin (0-200 μg/ml), gentamycin (0-100 μg/ml), tetracycline (0-50 μg/ml). Cell density was measured at 600 nm after incubation at 37°C for 12-16 hours. |
| Identification and characterization of isolated strains |
| For 16S rRNA sequencing analysis, genomic DNA was isolated by CTAB method [33]. The 16S rRNA sequence was amplified using universal bacterial primers 27F (5’-AGAGTTTGATCCTGGCTCAG-3’) and 1492R (5’-GGTTACCTTGTTACGAC TT-3’). The 50 μl PCR reaction system contained 1 μl 20 μΜ 27F and 1 μl 20 μΜ 1492R, 2.5 U/μl Taq DNA polymerase (0.3 μl) (TIANGEN Inc., China), 5 μl 10×PCR buffer (TIANGEN), 4 μl 2.5 mM deoxynucleoside triphosphate (TIANGEN) and H2O. Temperature cycling comprised 30 cycles of 94°C for 1 min, 50°C for 1 min and 72°C for 2 min. Purified PCR products were cloned in pMD18-T vector and transferred into E. coli strain DH5α. The 16S rRNA gene of clones was sequenced at ShangHai Majorbio Bio-pharm Technology Co. Ltd and sequences were analyzed using NCBI database and EzTaxon database to identify the most closely related organisms. Phylogenetic trees were built by MEGA and Treeview program. |
| Transmission electron microscopy (TEM) analysis |
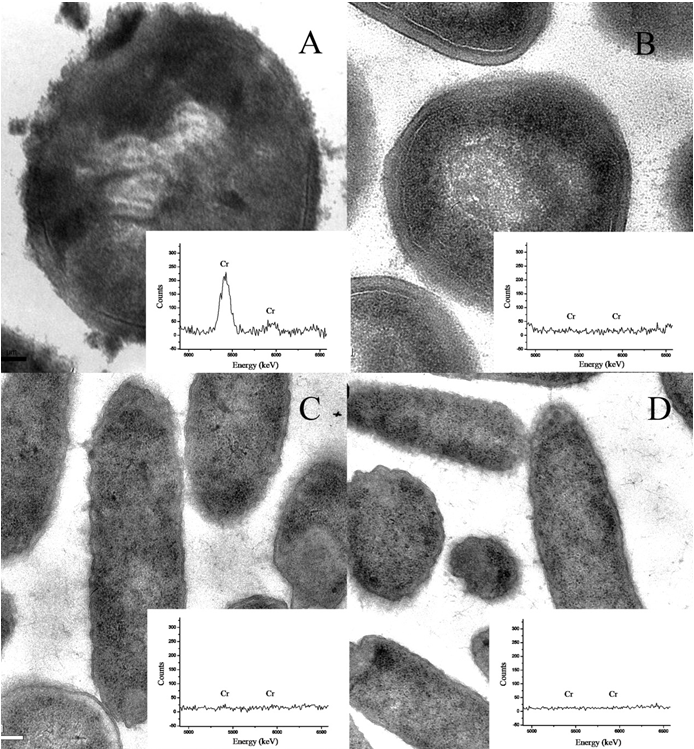
| LZ-01 was cultivated in M9 minimal salts medium with 1 mM Cr(VI) at 37°C, 180 rpm for 48 hours. Cells were harvested by centrifugation (10000×g) at 4°C for 3 min and resuspended in deionized water and washed twice. Cells were resuspended in 2.5% (v/v) glutaraldehyde in phosphate buffered saline (PBS) for at least 2 hours. Then cells were washed 3 times in PBS followed by fixation with 1% (w/v) osmium tetroxide for 2-3 hours and washing three times with PBS. Samples were gradually dehydrated in 50%, 70%, 90%, and 100% (v/v) ethanol for 10 min each. After incubation in the mixture of epoxy resin and acetone (v/v=1/1 for one hour, v/v=3/1 for 3 hours) and pure epoxy resin overnight, samples were embedded in solid resin blocks at 70°C for 24 hours. Blocks were sectioned on a microtome and ultrathin sections were observed by using TEM (Tecnai™G2F30, FEI. USA). Isolated strains grown in M9 medium without Cr(VI) and E. coli strain DH5α grown in M9 medium with and without Cr(VI) were prepared as controls. |
| Phylogenetic analysis |
| LZ-01 was pre-cultured by growing in M9 medium overnight and then streaked on an M9 plate in four zones. Approximately 40 mg (wet weight) of cells was harvested from the third zone. The whole cell Fatty Acid Methyl Esters (FAMEs) were extracted by the Microbial Identification System (Microbial ID, Inc., Newark, Del.) and analyzed by gas chromatography. The Microbial Identification System Standard Software was used to integrate peaks and determine fatty acid identity and concentration. S. aureus strains ATCC 25923 and ATCC 29213 were used as controls. |
| Physiological tests were done using the Vitek 2 System (bioMérieux Industry, France). Strain LZ-01 was examined with the colorimetric identification GP card which tested 64 different carbon sources as well as enzyme activities and antibiotic resistance. The test was performed according to the manufacturer instructions for 8 hours. S. aureus strains ATCC 25923 and ATCC 29213 were tested in the same way as standard strains. |
| Results |
| Cr(VI) reduction of isolated strains |
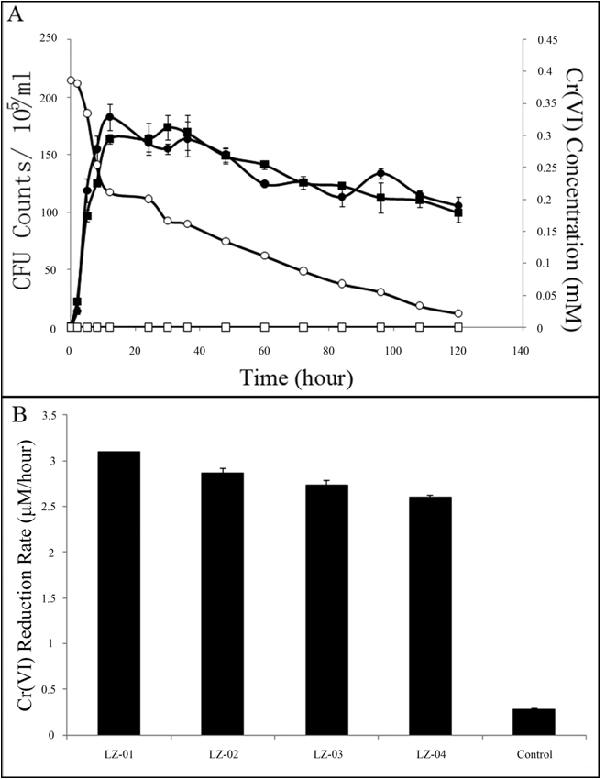
| Thirteen Cr(VI)-resistant strains capable of growing with 2 mM Cr(VI) in liquid M9 media were isolated from the soil samples. The ability of four strains (LZ-01, LZ-02, LZ-03, LZ-04) to reduce Cr(VI) while growing with 0.4 mM to 9 mM Cr(VI) was investigated. Different concentrations of Cr(VI) had the similar effects on cell growth (Supplementary Figure 1A). Since LZ-01 showed the highest reducing ability, it was chosen for further study. Within 60 hours LZ-01 reduced 67.89%, 10.35%, 7.02%, 7.54%, 8.42% and 3.51% with 0.4 mM, 1 mM, 2.5 mM, 5 mM, 7.5 mM and 9 mM Cr(VI), respectively (Figure 2A and Supplementary Figure 1B). As reduction appeared to be most efficient at 0.4 mM Cr(VI), we chose this concentration for further reduction experiments. Similar results were obtained with strains LZ- 01, LZ-02, LZ-03, LZ-04 with 94.5%, 89.1%, 88.6%, 85.2% of 0.4 mM Cr(VI) reduced respectively after 120 hours’ incubation (Figure 2B). The Cr(VI) reduction rate of LZ-01 was 3.10 μM/hour and the bacterial doubling time with Cr(VI) was 5.61 hours. When the growth curve was compared with control, it showed that 0.4 mM Cr(VI) did not repress the growth of LZ-01 (Figure 2A). |
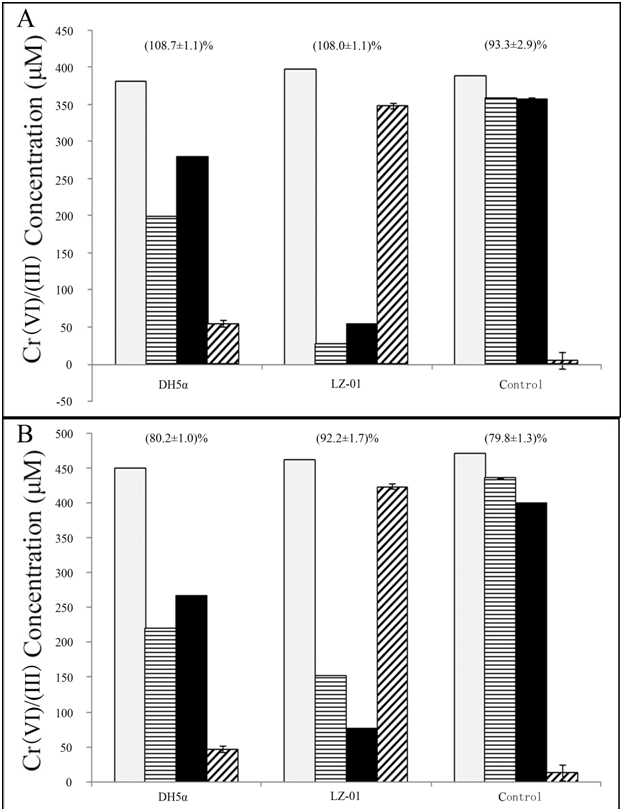
| Total hexavalent chromium level in the culture during the incubation with Cr(VI) was measured in growing cells and washed cells. E. coli strain DH5α and strain LZ-01 were both grown in 0.4 mM Cr(VI) M9 media. The soluble and sorbed Cr(VI) concentrations were measured before and after 120 h incubation (Figure 3A). Results showed that the E. coli strain DH5α removed 182 μM Cr(VI), of which 80 μM was sorbed Cr(VI). The recovery rate of Cr(VI) was about 75% of the initial Cr(VI) added. This data indicated that the decrease of Cr(VI) within the E. coli culture was mainly a result of biosorption. On the other hand only 27.1 μM Cr(VI) was sorbed in LZ-01 cells, and total 369.5 μM Cr(VI) was removed from LZ-01 culture with about 5.5% of the initial Cr(VI) remaining, which suggested that the decrease of Cr(VI) in LZ-01 culture was mainly due to reduction. In addition, the presence of a much higher level of Cr(III) in the LZ-01 culture provided clear evidence that chromate reduction occurred (Figure 3A). The combined concentration of reduced Cr(VI), biosorbed Cr(VI) and remaining dissolved Cr(VI) almost equaled to the initial Cr(VI) concentration. Meanwhile, control medium without bacteria showed no change in Cr(VI) concentration. This confirmed that the Cr(VI) decrease was a biotic process. Similar results were obtained in the washed cells (Figure 3B). |
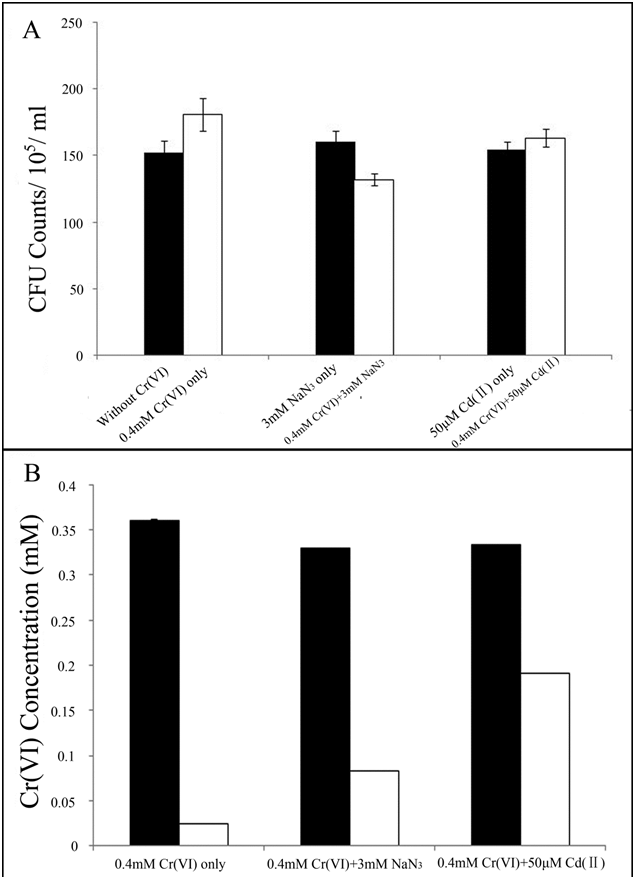
| Previous studies showed that microbial Cr(VI) reduction can take place on cell membrane [34] as well as in cytoplasm [35]. To determine whether the Cr(VI) reduction was associated with extracellular or surface associated proteins, NaN3 and Cd(II) were added to reduction experiments. NaN3 is an inhibitor of microbial respiratory chain [36] and Cd(II) inhibits the proteins that make use of thioredoxin [35]. CFU counts were used in this test to evaluate the toxicity of Cd(II) and NaN3 to living cells. Growth was not significantly influenced by the addition of either NaN3 or Cd(II) (Figure 4A). The results showed that NaN3 treatment inhibited Cr(VI) reduction in LZ-01 by 26.5% and Cd(II) treatment repressed the process by approximately 42.6% (Figure 4B) suggesting that Cr(VI) reduction in LZ-01 may require both components of the respiratory chain and cytoplasmic molecules that are sensitive to Cd. Control group in supplementary figure 2 showed that M9 medium had no effect on the measurement of Cr(VI) concentration. |
| Optimal growth conditions of LZ-01 |
| Determination of growth curves and CFUs under a range of pHs showed that the growth was optimal at pH 7 (Figures 5A and 5B). The temperatures tested range from 20°C to 42°C and the growth of LZ-01 peaked at 37°C (Figure 5C and 5D). Therefore, pH 7 and 37°C was the optimum condition for LZ-01 growth and reduction with Cr(VI). |
| LZ-01 resistance to As(V), Hg(II), U(VI), Cr(VI) and to antibiotics |
| As Cr(VI) contamination is often associated with other metals including uranium (VI) and arsenic (V) [35,37,38], we tested the tolerance of strain LZ-01 to As(VI), Hg(II), U(VI) and Cr(VI). Strain LZ-01 showed robust resistance to Cr(VI), As( V), and U(VI) (9 mM, 6 mM and 10 mM respectively), but not Hg(II) (MIC 0.025 mM) (Table 1). LZ-01 showed varied resistance to different antibiotics (Table 2). |
| Localization of reduced Cr(VI) in LZ-01 identified by TEM |
| To determine whether the reduction of Cr(VI) to Cr(III) occurred intra- or extracellularly, we used TEM to identify the presence of Cr(III) precipitate in LZ-01 culture (Figure 6). The TEM images showed that LZ-01 cells were all coccus-shaped and had smooth membrane which was consistent with typical S. aureus cells. The precipitate of Cr(III) was detected by EDX analysis after incubation while no Cr- containing material were observed in the negative control (Figure 6). Cr(III) precipitates were detected both free in the cells and adhering to the cell membranes suggesting that the reduction of Cr(VI) occurred both intracellular and extracellular. On the other hand, the images of E. coli strain DH5α showed no Cr(III) precipitates in cells with or without Cr(VI) treatment. This result suggest that reagents used during TEM sample preparation are not to able to reduce Cr(VI). |
| Phylogenetic analysis of isolated strains |
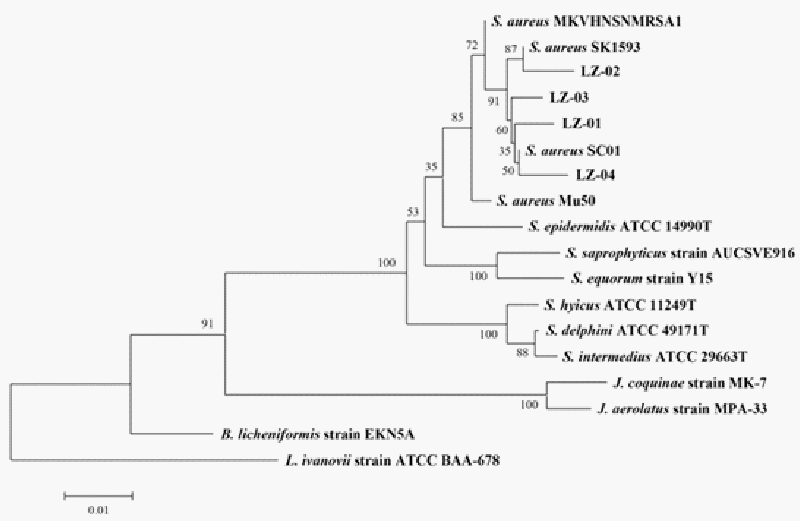
| Morphological characterization and 16S rRNA gene sequencing were carried out to identify four isolates (LZ-01, LZ-02, LZ-03, LZ-04). All four isolates were coccus-shaped, approximately 1 μm in diameter, and Gram-positive. The 16S sequencing data indicated that all four strains were closely related to S. aureus. LZ-01 was 98.5% similar to S. aureus subsp. anaerobius. LZ-02 was 98.1% similar to S aureus subsp. aureus. LZ-03 was 99.0% similar to S. aureus subsp. anaerobius. LZ-04 was 98.8% similar to S. aureus subsp. aureus (Figure 7). |
| Whole cell fatty acid was analyzed following the standard protocol of MIDI [39]. The fatty acid profiles of LZ-01, LZ-02, LZ-03, LZ-04 and S. aureus type strains ATCC29213 and ATCC25923 [40,41] were presented in supplementary figure 3. The major fatty acids of the strains tested were C15:0 iso, C15:0 anteiso, C16:0, C17:0 iso, C17:0 anteiso and C18:0. This result was similar to the type strains of S. aureus confirming that all these four strains were closely related to S. aureus. |
| To further clarify the relationship between LZ-01 and S. aureus, we conducted Vitek phenotypic identification of strain LZ-01 and strains ATCC 25923 and ATCC 29213. The result showed that LZ-01 used 57 out of 64 of the same carbon sources with the two type strains. However, LZ-01 still displayed some major differences in comparison with the S. aureus type strains. LZ-01 produced α-glucosidase during growth while the other two type strains did not; LZ-01 could not utilize polybutylene and D-maltose while type strains could (Supplementary Figure 4). This data further confirmed that LZ-01 is closely related to S. aureus but still possesses significant differences from any known S. aureus strains. |
| Discussion |
| In this study, we screened surface sediment of Lanzhou reach of the Yellow River for Cr(VI)-reducing microbes. Thirteen hexavalent chromium-resistant strains were isolated. Four of them grew with up to 9 mM Cr(VI) and reduced Cr(VI) to Cr(III). All four isolates were closely related to S. aureus. Strain LZ-01 reduced Cr(VI) while growing aerobically and this activity appeared to be associated with both intracellular and extracellular activities. Cellular fatty acid and carbon source profiling suggested that LZ-01 is a novel subspecies of S. aureus, a result that was also supported by the transcriptome analysis. |
| Many Cr(VI)-reducing bacteria have been isolated from Cr(VI)- contaminated sites [30,31,44]. Many of these studies looked at microbial Cr(VI)-reducing processes in anaerobic environment and found enzymes involved to be oxygen sensitive [34,36]. However, many Cr(VI)-contaminated aquatic environments are aerobic including Lanzhou reach. Anaerobic Cr(VI)-reducing bacteria may not be suitable for microbial remediation in such backgrounds. There are a few known strains that are able to reduce Cr(VI) aerobically, but their activity may not be as strong as anaerobic Cr(VI) reduction [43-46]. For example, E. coli produces a nitroreductase (NfsA) that has been shown to reduce Cr(VI) [44]. However, it is still sensitive to Cr(VI) and its ability to reduce Cr(VI) is limited (Figure 3). |
| Cr(VI) tolerance of strain LZ-01 is higher than any known aerobic Cr(VI) reducers and many anaerobic Cr(VI) reducers including Desulfovibrio vulgaris [34] and Desulfovibrio desulfuricans G20 [35]. Since LZ-01 was able to aerobically reduce Cr(VI) efficiently, it might be a good candidate for Cr(VI) remediation in Lanzhou reach. |
| Mechanisms of microbial Cr(VI) reduction can be divided into extracellular and intracellular reduction. In some previous studies, aerobic Cr(VI) reduction has been shown to occur intracellularly [44,45]. A cytoplasmic metal reductase in Desulfovibrio desulfuricans G20 reduces Cr(VI) using thioredoxin as electron donor [35]. However, Desulfovibrio vulgaris is known to reduce Cr(VI) in the periplasm with the involvement of cytochrome c [34]. In LZ-01, Cr(III) precipitates were detected both within and outside of cells (Figure 6) indicating that the reduction involved both intracellular and extracellular processes. Cd(II) is an inhibitor of thioredoxin and it repressed 42.6% of Cr(VI) reduction in LZ-01. NaN3, a cytochrome oxidase inhibitor, inhibited only of 26.5% Cr(VI) reduction (Figure 4). These results suggest that these inhibitors lack specificity for the target proteins. But they also suggest that there might be two independent mechanisms of Cr(VI) reduction, an interpretation that is backed up by the results from TEM analysis. |
| Although S. aureus is frequently isolated from the environment [11,27-29,47], it has not previously been shown to reduce Cr(VI). A S. aureus plasmid pI258 has been shown to encoded a gene CadC that contains two distinct metal-binding sites for several metals [29], but not Cr(VI). A few other Staphylococcus strains have been reported to reduce Cr(VI). S. epidermidis L-02 can reduce Cr(VI) under anaerobic condition [11] and Staphylococcus xylosus can biosorb Cr(VI) and Cd(II) in single ion and binary solutions [47]. |
| Strain LZ-01 is also resistant to As(V) and U(VI) (Table 1) but sensitive to Hg(II). Cr(VI), U(VI) and As(V) are all high valence oxidized metals existing in anions Cr2O7 2-, UO22+ and AsO43- while Hg(II) is in the cation form Hg2+. It is possible that the resistance mechanisms for Cr(VI), U(VI) and As(V) are similar in LZ-01. Several oxidoreductases have been shown to reduce both U(VI) and Cr(VI). e.g. ChrR and NfsA [44,46]. These proteins obtain electrons from NADH/NADPH and transfer two electrons to Cr(VI) or U(VI). Both ChrR and NfsA are cytoplasmic proteins and strain LZ-01 may have a similar mechanism for the reduction of these metals. Desulfovibrio desulfuricans G20 is also known to tolerate Cr(VI), U(VI) and As(V) with a common mechanism for both Cr(VI) and U(VI) reduction [35,48]. |
| Metal-resistant microorganisms are often presented in contaminated soils [49-54]. These bacteria are already well adapted to the local environment and they will likely have a competitive advantage over allochthonous strains if introduced for bioremediation. Lanzhou reach of the Yellow River is heavily contaminated with metals, threatening downstream population with bioremediation occurring near the source of the metals. Hence, S. aureus may play an important role in Cr(VI) remediation at this site. |
| Acknowledgements |
| Funding for this work was supplied by grants from Lanzhou University, lzujbky-2011-32 and from NFSC 31200085, due to X, Li. We sincerely thank Dr. Chengxiang Fang from Wuhan University for helping on phylogenetic analysis on strain LZ-01 and Dr. Xiaoning Hu from Gansu CDC for helping on Vitek analysis. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
 |
 |
 |
| Figure 5 | Figure 6 | Figure 7 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 15031
- [From(publication date):
May-2013 - Mar 29, 2025] - Breakdown by view type
- HTML page views : 10266
- PDF downloads : 4765
