Research Article Open Access
A New Synthesis of Nickel 12-Hydroxy Oleate Formulation to Improve Polyolefin's Degradation
| Anniyyappa Umapathi Santhoskumar, Komaragounder Palanivelu*, Shailendra Kumar Sharma and Sanjay Kumar Nayak | |
| Department of Plastic Technology, Central Institute of Plastic Engineering and Technology, Guindy, Chennai-600032, India | |
| Corresponding Author : | K.Palanivelu Department of Plastics Engineering & Technology TVK Industrial Estate, Guindy Chennai – 600 032, India Tel: +919677123881, +914422254708 Fax: +914422251707 E-mail: kpalanivelucipet@gmail.com, santhosannauniv@gmail.com |
| Received August 31, 2010; Accepted October 20, 2010; Published October 23, 2010 | |
| Citation: Santhoskumar AU, Palanivelu K, Sharma SK, Nayak SK (2010) A New Synthesis of Nickel 12-Hydroxy Oleate Formulation to Improve Polyolefi n’s Degradation. J Bioremed Biodegrad 1:108. doi: 10.4172/2155-6199.1000108 | |
| Copyright: © 2010 Santhoskumar AU, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
A new additive Nickel (Ni) 12-hydroxyoleate was successfully synthesized and their performance on the photodegraded low density polyethylene and polypropylene fi lms were subjected to biodegradation in the presence of the microbes such as Aspergillus niger and Pencillium funculosum isolated from a dump. Fragments occur progressively in the biodegradation of the photodegradaded fi lms. Moreover, the biodegradation test results reveal that 19% and 23% respectively of the material degradation at the end of 45 days. This Ni 12-hydroxyloleate blended with LDPE and PP fi lms has been exposed to abiotic and biotic environments. The abiotic degradable of the fi lms were UV irradiated for periods of maximum within 96 hours of LDPE and 72 hours of PP in different percentage before being mixed with water and organic fraction municipal solid compost were examined by infrared spectroscopy. The carbonyl peak increased with time in the abiotic environment and the oxidative degradation. In the presence of a biotic environment however, this peak decreased. At the same time there was an increase in double bonds which was related to weight loss. This mechanism is compared, on the one hand, with abiotic photo-oxidation, Norrish type I degradation and on the other with biotic polyole fi n’s degradation to produce double bond formation fi nd out peak in FTIR. So it is proxidante and bioactive LDPE and PP-Ni 12-hydroxyloleate degradable simply. The SEM micrograph con fi rms the presence of the deterioration of fi lm increases with increase of percentage additive due to the presence of microbial exposure.
| Keywords |
| Biodegradation; Biotic environment; Abiotic environment; Low-density polyethylene; Polypropylene; Nickel 12-hydroxy oleate |
| Introduction |
| The solution of plastic ecological problem lies in the development of photodegradable and biodegradable polymer with controlled lifetime. The additives like Ketones, quinones and peroxides are initiators of photo-degradation reactions [1-11]. |
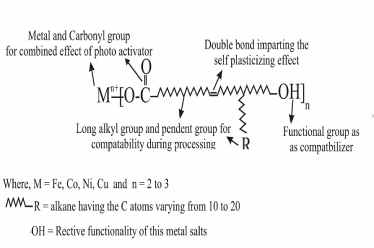
| Polyolefin’s is relatively inert due to its hydrophobic chain and high molecular weight. So degradability offers a complimentary strategy to deal with this waste problem. One of the simplest ways of modifying the existing polymer is to accelerate the degradation process already taking place. Different approaches to develop photo degradable polyolefin have been adopted, including both co-polymerization with ketene or CO groups and addition of photo initiating metal complexes such as transition metal 12-hydroxy oleate as shown in Figure 1 and its reaction Figure 2. Photo-oxidation leads to an increase in the low molecular weight fraction by chain scission, thereby facilitating biodegradation. It also leads to an increase in the surface area through embrittlement. In addition, the formation of carbonyl groups on the surface increases its hydrophilicity. Consequently, the possibility of further degradation induces a significant enhancement towards mineralization of plastic material. |
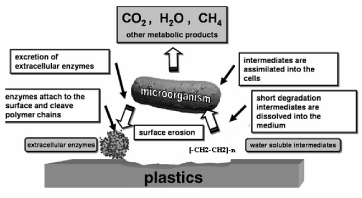
| The photodegraded films to evaluate biodegradation using microorganisms such as Aspergillus niger and Pencillium funculosum are involved in the degradation of both natural and synthetic plastics. As shown in Figure 3. The biodegradation of plastics proceeds actively under various organic fraction solid compost or municipal solid waste conditions according to their properties, because the microorganisms responsible for the degradation differ from each other and they have their own optimal growth conditions in the organic fraction of solid compost. Polymers especially plastics are potential substrates for heterotrophic microorganisms. Biodegradation is governed by different factors that include polymer characteristics, type of organism, and nature of pretreatment. The polymer characteristics such as its mobility, tacticity, crystalline, molecular weight, the type of functional groups and substituent’s present in its structure, and plasticizers or additives added to the polymer all play an important role in its degradation. During degradation the polymer is first converted to its monomers, and then these monomers are mineralized. |
| Nickel 12-hydroxyloleate within the polyolefin such as Polyolefin’s this additive most polymers are too large to pass through cellular membranes, so they must first be depolymerized to smaller monomers before they can be absorbed and biodegraded within microbial cells than to the virgin Polyolefin’s. The initial breakdown of a polymer can result from a variety of physical and biological forces physical forces, such as heating or cooling, freezing or thawing, or wetting or drying, can cause mechanical damage such as the cracking of polymeric materials. The growth of many living (fungi) can also cause small-scale swelling and bursting, as the fungi penetrate the polymer solids. |
| High molecular weights result in a sharp decrease in solubility making them unfavorable for microbial attack because bacteria require the substrate to be assimilated through the cellular membrane and then further degraded by cellular enzymes. At least two categories of enzymes are actively involved in biological degradation of polymers: extracellular and intracellular depolymerases during degradation, exoenzymes from microorganisms break down complex polymers yielding smaller molecules of short chains, g., oligomers, dimers, and monomers, that are smaller enough to pass the semi-permeable outer bacterial membranes, [13] and then to be utilized as carbon and energy sources. The process is called depolymerization. When the end products are CO2, H2O, or CH4, the degradation is called mineralization. It is important to note that biodeterioration and degradation of polymer substrate can rarely reach 100% and the reason is that a small portion of the polymer will be incorporated into microbial biomass, humus and other natural products dominant groups of microorganisms and the derivative pathways associated with polymer degradation are often determined by the environmental conditions. When O2 is available, aerobic microorganisms are mostly responsible for destruction of complex materials, with microbial biomass, CO2, and H2O as the final products. |
| Experimental |
| Materials and methods |
| Ammonium Nickel (II) sulphate hexahydrate, sodium hydroxide, Ricinoleic acid were used without further purification. General purpose film grade LDPE 24FSO40 and PP H034SG has been used to prepare films. Milli Q ultrapure water was used throughout the course of this work. Ba(OH)2 and hydrochloric acid SQ qualigens in Fisher Scientific. |
| Synthesis and preparation of Nickel 12-hydroxyl oleate |
|
1 mole of NaOH is mixed with 1000 ml of ethanol in a volumetric flask to get 1N NaOH solution. The required amount of NaOH solution is prepared in a volumetric flask. 720 ml of 1N NaOH and 80 ml of fatty acid are taken in a round –bottmed flask and refluxed for one hour. Porcelain pieces are put inside the round-bottomed flask to avoid spitting out of solution. Condensed is attached and continuous supply of water is provided during reflux. Cotton plug is kept on the neck of the condenser to avoid evaporation. 100 ml of 15 N HCl is mixed in 1400 ml of distilled water in beaker to get 1N HCl.1 mole of NaOH was dissolved in 1000 ml of distilled water to get one mole aqueous NaOH solution. 60 g of the collected mixture was dissolved in 200 ml of 1mole aqueous NaOH solution and as a result a single phase solution of sodium salt is got. 46 g of (Nickel ammonium sulphate) FAS is dissolved in required amount of water to get one mole of FAS solution. One mole of FAS solution was mixed with water several times till a pure water layer is separated. Then the remaining mixture is washer with a small amount of ethanol and taken in a there resulting mixture is called the Ni 12-hydroxyl oleate. |
| Blending and film preparation of LDPE |
| LDPE was blended with synthesized Ni 12-hydroxyl oleate additive in varies percentage 1%,3% and 5% by using Torque Rheometer, blending was carried out at a temperature range of 130-190°C and at a screw speed of 75 rpm. Subsequently, the pellets are dried in a dehumidifier at 70°C for two hours to remove moisture. The film was prepared by using film die for all the three percentage of additive. The three percentages of additives. The wall thickness of the film was kept at 50 microns by controlling the speed of the nip rollers and output rate. |
| Blending and film preparation of PP |
| The Ni 12-hydroxyl oleate was melt blended with PP at three different formulations 1, 2 & 3% respectively in Torque Rheometer. Blending was carried out at temperature range of 210,200, 190,180 and 150°C (From die to hopper) and a screw speed of 100 rpm. Subsequently, the pellets are dried in a dehumidifier at 70°C for two hours to remove moisture. The pellets produced were subsequently dried and subjected to film cast process to produce films of 50 microns thickness. |
| Polyethylene and Polypropylene was blended with the synthesized Ni 12-hydroxyoleate additive containing double bond acting as plasticizing effect, hydroxyl group as compatibilizer, long alkyl group and pendent group for compatibility during processing and metal and carbonyl group for combined effect of photo activator by UV radiation in polyethylene and polypropylene films. This biological activity of transition metal 12-hydroxyoleate containing functional group as multifunctional active. This may represented as multifunctional active additive (MFA) as shown in Figure 1. |
| The above Nickel (Ni) 12-hydroxy oleate was successfully synthesized and their performance on the photodegradation of (QUV Accelerated weathering tester model QUV/spray with solar eye irradiative control) low density polyethylene and polypropylene films were subjected to biodegradation in the presence of the microbes such as Aspergillus niger and Pencillium funculosum isolated from a dump. The above mentioned film ASTM D 5338-98 test method determine the degree and rate of aerobic biodegradation of plastic materials on exposure to a controlled- composting environment under laboratory conditions. This test method is designed to yield reproducible and repeatable test results under controlled conditions that resemble composting conditions. The test substances are exposed to inoculums that are derived from compost from municipal solid waste. The aerobic composting takes place in an environment where temperature, aeration and humidity are closely monitored and controlled. |
| Elemental analysis |
| The carbon content of the each test sample determined by elemental analysis by using Carlo Erbal model 1106 elemental analysis. |
| Biodegradation of polyolefin’s evaluated from the blank and sample vessels based on ASTM 5338-98 compost biodegradation |
| The method used for the determination of the biodegradability of the polyolefin’s was based on the International Standard (ASTM 5338-98) that measures the evolved CO2 amount from both the blank vessel without a sample and the sample vessel including a 10g polyolefin’s sample, 600g mature solid compost The newly developed biodegradation measurement system using the compost biodegradation apparatus with the absorption columns is shown in Figure 4. This evaluation system for the biodegradation uses the CO2 trap system with CO2 absorption columns. This compost biodegradation mechanism is as follows. First, room air is passed into the carbon dioxide trap to remove the CO2 in the air. This air is moisturized and passed into the reaction vessel controlled at 58±2°C. The air with the produced CO2 from the biodegradation of the samples and respiration of the microorganisms in the solid compost is passed into the Ba(OH)2 aqueous solution to remove the produced BaCO3 from the compost to obtain an accurate carbon dioxide using a titration method [14]. |
| Conventional CO2 evolution test |
| The principle of the widely used CO2 evolution test (ASTM 5338- 98) was the determination of the ultimate biodegradability of organic compounds by aerobic microorganisms, using a static aqueous test system and the evolution of CO2 as the analytical parameter. A 1.50-liter test mixture was prepared in 2-liter vessels containing an inorganic medium and the organic compound as the sole source of carbon at a concentration of 10 to 40 mg of organic carbon liter-1. Usually organic fraction solid compost, obtained from a wastewater treatment plant or from another source in the environment, was used as mixed inoculums. The vessels were aerated with 1 to 2 bubbles of CO2-free air per sec (50 ml min-1) and incubated at 58 ± 2°C for usually minimum 45 days. The bigamous CO2 formed during the microbial degradation was trapped in two external adjacent vessels (volume, 1500 ml) containing an aqueous Barium hydroxide Ba(OH)2 solution (0.25 N). Samples were taken at regular intervals to determine the amount of dissolved inorganic carbon (DIC) and to calculate the amount of CO2 produced with titrated Hydrochloric acid this evolved CO2 was compared with the calculated theoretical amount (Th CO2), and the degree of biodegradation was expressed as a percentage. |
| Titration method of CO2 determined test |
| The degradation of chemical compounds in the titration CO2 evolution was based on the same principle as the conventional CO2 evolution test. Test vessels were cylindrical bottles with a 2-liter volume containing the same mixture of inorganic (Barium hydroxide) medium, organic test substance, and mixed inoculums in a liquid volume of 1.5 liter. Incubation, aeration with CO2-free air and agitation of the test mixture, blank and control vessels were also comparable. The exhaust gas from the vessels was passed through a glass chamber (volume, 1500 ml) that was immersed in the test mixture and filled with 50 ml of a 0.25 N aqueous Ba(OH)2 solution titrated with HCl after the end point remain Barium Hydroxide calculated and subtracting the blank values, biodegradation was calculated and expressed as a percentage of the CO2. There was a linear correlation between the amounts of carbon dioxide liberated. This correlation was determined for each volume and concentration of absorption solution used. |
| Oxygen a llowed complete oxidative biodegradation of the test compound. The concentration of inoculums was 30 mg of dry matter of Solid compost and the concentration of the test compound was 100 mg of substance liter-1 or 50 to 100 mg liter-1 of theoretical oxygen demand (ThOD). The test duration was minimum 45 days and the tests were performed at an incubation temperature of 58±2°C [14]. |
| ASTMD-5338: Test procedure |
| The amount of CO2 produced pass through the Ba(OH)2 Solution and is precipitated as Ba CO3 |
| Ba(OH)2 +CO2 –> BaCO3+H2O |
| Determine the CO2 by titrating the remaining Ba(OH)2 with 0.05N HCl |
| Ba(OH)2 +2HCl –> Ba Cl2+2H2O |
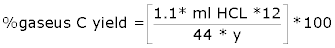 |
| Y=mg carbon charged to flask |
| Biodegradation Calculation |
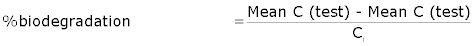 |
| Where; |
| Cg= amount of gaseous carbon produced g and |
| Ci= amount of carbon in test compound in the sample,g. |
| Scanning electron eicroscopic analysis (SEM) |
| The scanning electron microscope analysis of fractured surface of LDPE and PP- Ni 12-hydroxyloleate films were carried out using CARL ZESIS Model; EVO MA 15 scanning electron microscope. The surface of the samples was coated with conductive heavy metal such as gold / palladium. |
| Fourier transform infrared spectrophotometer (FTIR) |
| The structural changes in LDPE and PP films in the presence of Ni 12-hydroxyl oleate after effect of UV exposure eight and three days respectively and then after microbial exposure for 45 days were studied by Nicolet 6000 (USA) Fourier Transform Infrared Spectrometer (FTIR) with the wave number range of 400-4000 cm-1. The degradation of the film was monitored with an IR instrument the films were cold drawn to make it possible to obtain transmission spectra. Samples to be analyzed by IR were cut out from the films and washed with distilled water to remove traces of organic fraction solid compost etc. In the IR spectra, special interest was focused on the following absorption peaks 1636 cm-1 double bonds (-C==C-) in this additive. |
| Thermal properties |
| Differential scanning calorimeter (DSC) analysis: Melting behavior of 12 hydroxyl oleate blended samples (LDPE and PP) after effect of UV exposure eight and three days respectively and then after microbial exposure were studied for 45 days by employing Perkin Elmer (USA) differential scanning calorimeter. Sample 2 mg weight were scanned from 45 to 200°C at the heating rate of 10°C/min to detect the melting characteristics of the sample before and after exposure to biotic environment. The percentage of crystallinity of 12 hydroxyl oleate blended LDPE and PP films were calculated as follows. |
| % of Crytallinity = (ΔHm-ΔHc)/ ΔHco |
| Where ΔHm- Enthalpy of melting (J/g) |
| ΔHc- Enthalpy of Crystallization (J/g) |
| ΔHCo-Enthalpy of 100 % crystalline polymer (277.3 J/g) |
| Thermo gravimetric analyses: Thermal degradation of LDPE and PP, Ni 12-hydroxyl oleate blended samples the effect of UV exposure five days and three day respectively and then after biotic environment films for 45 days were analyzed by Perkin Elmer (USA), at the heating rate of 10°C/min from 50 to700°C. |
| Results and Discussion |
| Elemental analysis |
| C, H, N elemental analysis reported in table below and then pure Nickel 12-hydroxy oleate. Nickel percentage 6.37% as shown in table 1. It has Carbon, Hydrogen and Nitrogen analysis only. Carbon values of cellulose dependent carbon and hydrogen analysis only independent of the oxygen in molecular structure. So it has carbon content 84% by the elemental analysis. |
| Biodegradation of the LDPE and PP with Ni 12-hydroxyl oleate |
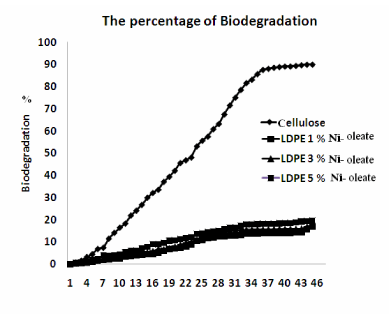
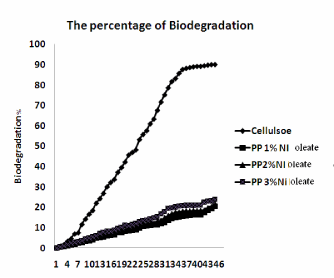
| Figure 5 and Figure 6 shows Conditions of reaction mixtures: Organ of compost; livestock excrement, municipal and Vegetable waste used the method used for the determination of the biodegradability of the polyolefin’s was based on the International Standard (ASTM 5338-98) that measures the evolved CO2 amount from both the blank vessel without a sample and the sample vessel including a 10g LDPE and PP, Ni 12-hydroxyl oleate samples. According to the experimental 2.9 ASTM 5338 test procedure. Polyethylene and polypropylene the percentage of biodegradation 19% and 23% respectively. |
| Reaction temperature : 58±2°C |
| Dry solid (%) : 52% |
| Volatile solid (%) : 19% |
| Air flow rate : 100 ml/min |
| Test duration (day) : 45 day |
| Reference material : Cellulose |
| Volume of reaction vessel : 2000ml |
| Moisture percentage in compost : 28 % |
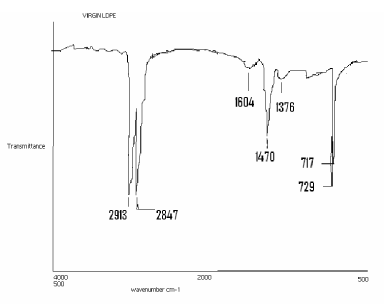
| Figure 8 shows the virgin LDPE samples to be analyzed by IR were cut out from the films. In the IR spectra, special interest was focused on the following absorption peak: 729 cm-1, rocking vibration (-CH2-); 1376 anti symmetric deformation(-CH3-); 1470 cm-1 symmetric deformation (–CH2-); 1604 cm-1, Symmetric deformation (–CH3-); 2913 anti symmetric deformation (–CH-); 2847 symmetric stretching (–CH3-). |
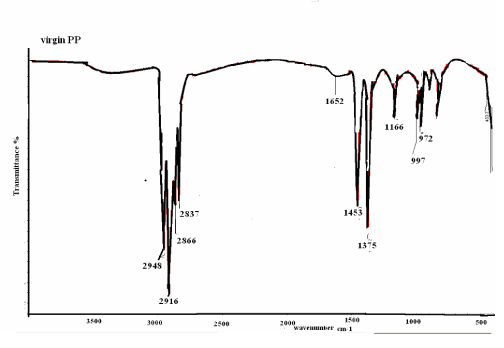
| Figure 10 shows the virgin PP samples to be analyzed by IR were cut out from the films. In the IR spectra, special interest was focused on the following absorption peak: 973 cm-1, rocking vibration (-CH2-); 997 cm-1, rocking vibration (-CH2-); 1167 cm-1 anti symmetric deformation (-CH3-); 1454 cm-1 symmetric deformation (–CH2-); 1167 cm-1, Symmetric deformation (–CH3-); 1167 anti symmetric deformation (–CH-); 2929 symmetric stretching (–CH3-). |
| Fourier transform infrared spectrophotometer (FTIR) |
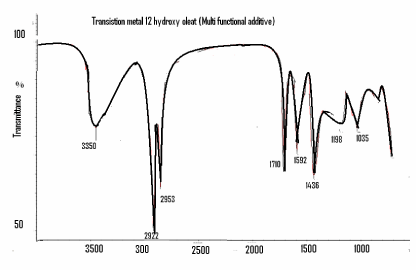
| Characterization of ferrous and nickel 12-hydroxy oleate: In Table 2 and in Figure 7, the FTIR spectra of the Ni 12-hydroxyl oleate exhibited absorbance at 1592 cm-1 due to asymmetric vibration stretching of the carboxylic group coordinated to the metal ion. The UV-Vis spectra of the oleate in ethanol show absorption maximum 290-305 nm as Ni 12-hydroxy oleate. |
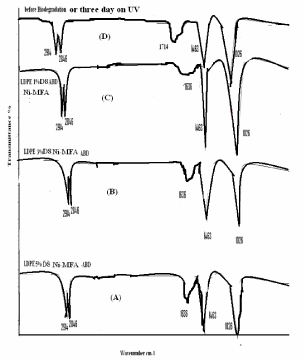
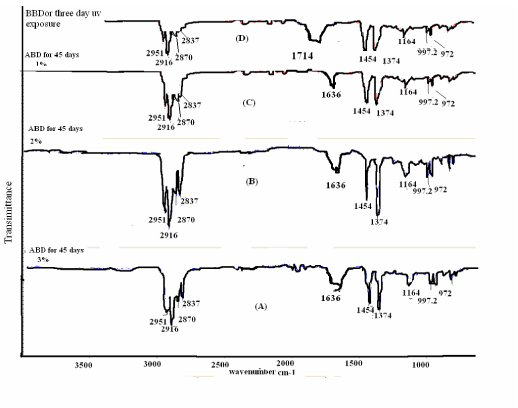
| Figure 1 showing the new class of generally structure of Ni 12-hydroxy oleate used for a study of the IR spectra of biotically degraded LDPE and PP. It was observed that the carbonyl index decreased with prolonged incubation time after the effect of UV exposure LDPE and PP- Ni 12-hydroxyl oleate for eight days and three days respectively. In Figure 9 and 11 (A,B and C) the explanation for this is given by the mechanism in some IR spectra of biotically degraded LDPE and PP a peak was noted corresponding to double bond groups1636 cm-1 in the additive. In Figure 9 and Figure 11(D) this peak was missing in abiotic (photo degradation) samples. In Table 3-6 differentiate FTIR spectra for virgin LDPE and PP compared to biotically degraded film from the photodegraded films. |
| The photodegraded films or Before biodegradation typical FTIR spectra as shown in Figure 9 and Figure 11(D) the measurement of carbonyl index 1714 cm-1, the conventional spectroscopic monitor of polymer degradation were made by measuring the IR transmission spectral of film after exposure for increasing lengths of time in QUV accelerated weathering equipment fitted with UVA -340 tubes and operated at 60°C the broad band radiation from the UVA-340 tubes has been shown to emulate sunlight in the wavelength range below 360 nm. The development of carbonyl groups was monitored and the carbonyl index, the absorbance at 1714 cm-1 was recorded. (As the film used in this study were 50µ thickness, the carbonyl index is numerically equivalent to the absorbance). |
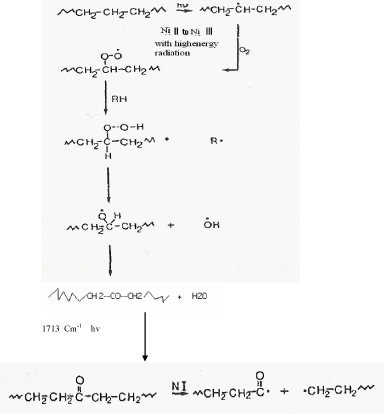
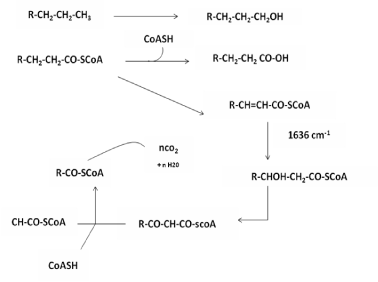
| Reference [15] is frequently made to Ni 12-hydroxy oleate films degradation by microorganisms when LDPE and PP its instability towards environmental factors are discussed. This is fairly understandable since a Ni 12-hydroxyl oleate can be classified as a very short-chain LDPE and PP, The length of typical Ni 12-hydroxyloleate, being 10-20 carbons chains. Microorganisms preferentially use linear carbon chain, whereas the corresponding branched isomers are almost completely inert to biodegradation. Figure 12 the mechanism for degradation of Ni 12-hydroxy oleate by microorganisms responsible for the attack on Ni 12-hydroxy oleate can be, for example, aspergillus niger and pencillium funculosum. |
| The polyethylene and polypropylene chain is oxidized to a carboxylic acid and the resultant acid undergoes β-oxidation which, by reaction with coenzyme A, removes two carbon fragments from the carboxylic molecule. The two carbon fragments, acetyl-SCoA, enter the citric acid cycle, from which carbon dioxide and water are released. From a Ni 12- hydroxyl oleate molecule with n carbons, a total of n molecule of carbon dioxide evolved [16]. |
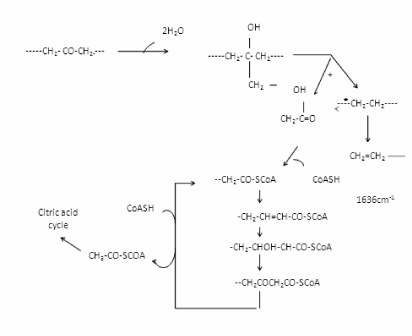
| A proposed mechanism for the biodegradation of LDPE and PP is given in Figure 13 it is thought that the alteration of LDPE and PP molecule is initially the same in both the abiotic (phtodegradation) and biotic samples. When carbonyl groups had been formed, the abiotic sample evidently did not undergo Norrish type II degradation as no double bond peak could be found in the IR spectra from abiotically (photodegradation) degraded LDPE and PP Figure 9 and Figure 11(D). According to the Figure 9 and Figure 11(D), the carbonyl index increased with prolonged incubation in an abiotic atmosphere. Alkenes formation had, however taken place, as was confirmed by the presence of peaks at 1636 cm-1 in biologically affected films. |
| Thermal properties |
| Differential scanning colorimetry: The differential scanning calorimetry data pertaining to the melting point and degree of crystallinity of Ni 12-hydroxyl oleate blended LDPE and PP film after the effect of UV exposure initiate the films and then after exposure biotic environment is presented in Table 7 and Table 8. |
| The virgin LDPE shows its melting point at 111.45°C. On the incorporation of Ni 12-hydroxyl oleate, the melting point is found to change slightly due to the presence of additive containing double bond as plastizing effect in LDPE matrix. After the effect of UV exposure or the photodegraded films LDPE, Ni 12-hydroxyl oleate samples exposed to biotic exposure for 45 days a marginal decrease in the melting point from 109.68 to 103.76°C was observed. This could be due to the new double bond formation (new peak formation find out in FTIR) by biotic exposure the biodegraded film. So it has faster biodegradation of LDPE films in the presence of Ni 12-hydroxyloleate additive. Corresponding ΔH peak get broadening indicates the formation of low molecular weight species due to biodegradation. The percentage of crystalline decreases by increasing the additive concentration. It was observed that the degree of crystallinity of LDPE films with 5 % Ni 12-hydroxyl oleate decreases from 70 to 58% due to the new double bond formation and the bond strength decreases through hydrolytic as well as oxobiodegradation during the biotic exposure and also act as plasticizing effect. These results are in agreement with the FTIR biotic degradation mechanism. |
| Similarly, in PP films with Ni 12-hydroxyl oleate the melting point data shows that the melting decreases by about 7°C with increasing the concentration of the additive from 1% to 3%. Also, the percentage of crystallinity decreases from 98 to 64% for 45 days in biotic environmental exposure. These results indicate that the PP films degrade faster than LDPE as it was expected due to the presence of methyl substituent groups in polypropylene. |
| Thermo gravimetric analysis (TGA): The thermo gravimetric analysis of LDPE and PP with Ni 12-hydroxyl oleate additive is summarized in Table 9 and Table 10. The results shows that the initial decomposition temperature of LDPE and PP blending with Ni 12-hydroxyl oleate decreases significantly after the effect of UV exposure for eight and three days respectively or before biodegraded films. After the biotic exposure, films LDPE and PP - percentage of initial decomposition temperature 42% and 0.08% respectively. This could be due to the new double bond formation and the bond strength decreases through hydrolytic and oxobiodegradation during the biotic exposure in ASTM D 5338. Decrease in initial decomposition temperature was observed with higher additive concentration than lower additive. This result does not confirm the biodegradability but initial decomposition temperature changes slightly after the effect biotic exposure films. |
| Scanning electron micrograph |
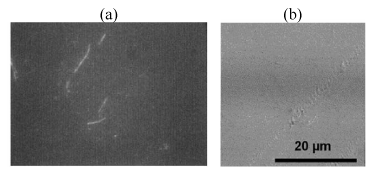
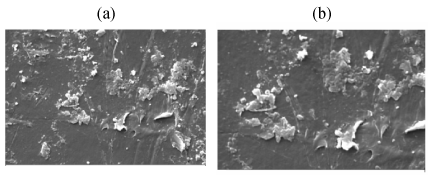
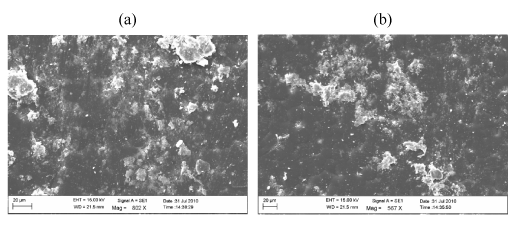
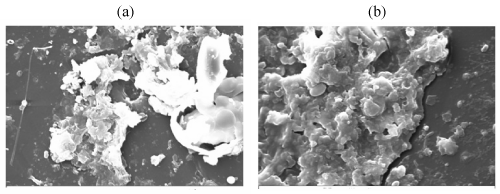
| A comparative compilation of scanning electron micrographs of two samples (LDPE and PP with Ni 12-hydroxyl oleate) at magnification of 500 to 2000. Figure 14 as is apparent from the surface of non degraded LDPE and PP is smooth, without fractured and free from defects. After the UV exposure both the sample compacted film leads to rapid disintegration of the surface for 15 and 45 days Figure 15-17(a, b) show typical pattern of microbial growth on the top surface of the sample. Erosion of the surface can be observed but interestingly, although microorganism grows fissure resulting from fracture propagation, none grow within the fracture suggesting that the most of the low molecular weight nutrient migrate to the surface from the (oxidized) layer of the polymer. It can be seen that the surface agglomerates were formed which could be due to the biodegradation involving deterioration of molecular chains. More surface agglomerations could be seen in the case of LDPE and PP, Ni 12-hydroxyl oleate indicating the faster rates of biodegradation. Also, the brittleness of the surface increases with increasing the exposure time and percentage of additive concentration. These results are in agreement with the FTIR mechanism as shown in Figure 12 and Figure 13. |
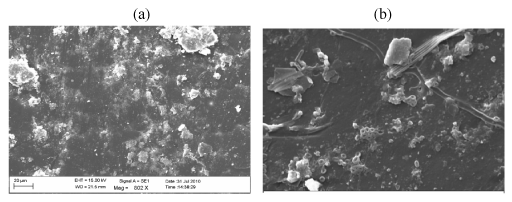
| Figure 18 shows the surface developed more crack and groove due to for 45 day biotic exposure for 3% Ni 12-hydroxyl oleate with LDPE and PP blend higher than 15 day biotic exposure. |
| Figure 19 shows the surface developed some crack and narrow grooves for 15 days by biotic exposure 3% Ni 12-hydroxyl oleate with LDPE and PP blends. |
| Figure 20 shows that the damage is much more pronounced in the sample containing Ni 12-hydroxy oleate with LDPE and PP blends after the biotic exposure for 45 days. |
| Conclusion |
| The rate of the new additive Nickel(Ni) 12-hydroxyoleate was successfully synthesized and their performance on the photodegraded low density polyethylene and polypropylene films were subjected to biodegradation of LDPE and PP film is very high at higher concentration of Ni 12-hydroxyl oleate. The LDPE and PP film containing 5% Ni 12-hydroxyl oleate has shown slightly changes degradation in thermal properties and reduction in degree of crystallinity on biotic exposure. After the biotic exposure in the FTIR analysis shows that a peak at around 1636 cm-1 corresponding to double bond group of transition metal 12 hydroxyl oleate was observed for LDPE and PP films with upto 5% and 3% additive respectively. The scanning electron micrographs of fractured surface of films after the biotic exposure show the brittle mode of fracture. The biotically degraded product when subject to biodegradation has results in 19% of LDPE and 23 % of PP biodegradation in 45 days. So, Ni 12-hydroxyl oleate is used as an effective biodegradable additive for LDPE and PP films. |
| Acknowledgements |
| The research was funded through The Department of Science and Technology sponsored CSIR project on “Technology development of biodegradable additive performance evaluation of biodegradation with various plastic” under grant number DST/TSG/WP/2006/58. We acknowledge discussions with our collaborators Dr. Rajeeve Sharma, Government of India, Ministry of Science & Technology, Technology Bhavan, New Delhi. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 | Table 5 |
| Table 6 | Table 7 | Table 8 | Table 9 | Table 10 |
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
 |
 |
 |
 |
 |
| Figure 6 | Figure 7 | Figure 8 | Figure 9 | Figure 10 |
 |
 |
 |
 |
 |
| Figure 11 | Figure 12 | Figure 13 | Figure 14 | Figure 15 |
 |
 |
 |
 |
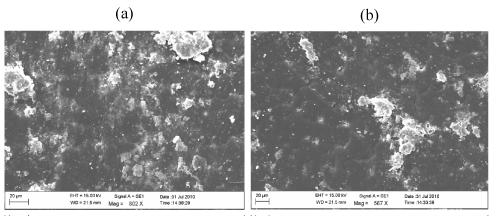 |
| Figure 16 | Figure 17 | Figure 18 | Figure 19 | Figure 20 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 16144
- [From(publication date):
November-2010 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 11447
- PDF downloads : 4697
