Research Article Open Access
A HPLC Method for Determination of Ursolic Acid and Betulinic Acids from their Methanolic Extracts of Vitex Negundo Linn
S. V. Taralkar1*and S. Chattopadhyay2
1Chemical Engineering Department, MAEER’s Maharashtra Academy of Engineering, Alandi (D), Pune-412105, Maharashtra, India
2Polymer Engineering Department, Indian Institute of Technology, Roorkee-247667, Uttarakhand, India
- *Corresponding Author:
- S. V. Taralkar
Chemical Engineering Department
MAEER’s Maharashtra Academy of Engineering
Alandi (D), Pune-412105
Maharashtra, India
Tel: +912030253621, +919011332500
Fax No: +912030253799
E-mail: suyogkumartaralkar@yahoo.co.in
Received date: April 23, 2012; Accepted date: June 01, 2012; Published date: June 07, 2012
Citation: Taralkar SV, Chattopadhyay S(2012) A HPLC Method for Determination of Ursolic Acid and Betulinic Acids from their Methanolic Extracts of Vitex Negundo Linn. J Anal Bioanal Tech 3:134. doi: 10.4172/2155-9872.1000134
Copyright: © 2012 Taralkar SV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Ursolic and betulinic acids are very useful nutraceuticals available in herbs. Their quantitative estimation from the solvent extracts of the herbs is a great challenge. So far all the chromatographic methods available for identification and quantification of these acids are distinctly different, meaning thereby, each acid would require a separate method. Reverse phase (RP-HPLC) method is developed for determination of ursolic acid and betulinic acid from their methanol extract of Vitex negundo Linn leaves. Analysis was carried out using Waters’ symmetry C-18 column with acetonitrile: methanol (80:20) as isocratic elution mode with UV detection (λ=210 nm). The method is pretty linear for ursolic acid in the range of 0.01-0.1 mg/ml (R2 = 0.9961) and for betulinic acid in the range of 0.003-0.018 mg/ml (R2 = 0.999). The peaks of ursolic acid and betulinic acid were confirmed by LC-MS. The method was validated by mixing these acids standards in methanol and found that it is accurate, sensitive and has a good reproducibility.
Keywords
Vitex negundo linn; Ursolic acid; Betulinic acid; HPLC; LC-MS
Introduction
Vitex negundo linn a member of Verbenaceae family, an important medicinal plant is found throughout India. In Hindi, it is called as “Nirgudi” or “Lagundi”. The extract from the leaves is the most important in the field of medicine [1]. The essential oils [2], β-sitosterol [3], Flavonoids [4] and triterpenoids- ursolic acid and betulinic acid [5] were isolated from the leaves and seeds of Vitex negundo linn. Ursolic acid is known to have anti-inflammatory, antitumor, antimicrobial, antibacterial and antifungal activity [6]. Ursolic acid (3β-hydroxy-urs-12-en-28- oic acid) is a pentacyclic triterpenoids. The physical properties of ursolic acid are: molecular formula C30H48O3, molecular weight 456.68 and melting point is 283-285°C. The structure of ursolic acid is as shown in Figure 1 [6].
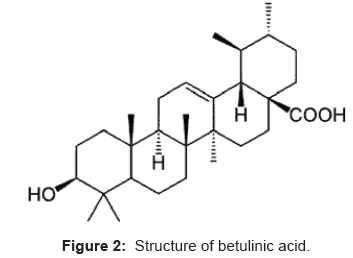
Betulinic acid (3β-hydroxylup-20-(29)-en-28-oic acid) is a pentacyclic triterpenoids [7]. Betulinic acid has been found to selectively kill human melanoma cells while leaving healthy cells alive. Due to its apparent specificity for melanoma cells, betulinic acid seems to be a more promising anti-cancer substance. Betulinic acid has also been found to retard the progression of HIV 1 infection, which eventually leads to AIDS, by preventing the formation of syncytia (cellular aggregates). In addition, betulinic acid has antibacterial properties and inhibits the growth of both Staphylococcus aureus and Escherichia coli [8]. Betulinic acid is not very poisonous, is relatively inexpensive. The physical properties of betulinic acid are: molecular formula C30H48O3, molecular weight 456.68 and melting point is 283-285°C. The structure of betulinic acid is as shown in Figure 2.
Determination of ursolic acid and betulinic acid content in the leaves of Vitex negundo linn can be obtained by various methods. HPLC is the one of the best and accurate method for determination of ursolic acid and betulinic acid in extract of Vitex negundo linn leaves. Many researchers have investigated and used different HPLC methods for determination of ursolic acid and betulinic acid separately in plant extracts. Simone et al. [9] used HPLC method with acetonitrile: water (70:30) as mobile phase, λ=203 nm wavelength with Novapack C-18 column to identify/estimate ursolic acid from Ilex paraguariensis aqueous extract. Hypersil C-18 column with methanol: water (95:5) as mobile phase and LC-MS detector was used by Liao et al. [10] to determine ursolic acid from Rat plasma. Ursolic acid from Perilla frutescens was estimated by Chen et al. [11] using RP-HPLC method with Sperisob Octadecylsilil Silica (ODS) column with acetonitrile and aqueous H3PO4 as mobile phase and UV detector (λ=206nm). Novotny et al. [12] analyzed methanol extract of Staphylea holocorpa Hemsl for ursolic acid content using LC-MS and Shim Pack CLC-CN, C-18 column and a mobile phase of methanol: 1% acetic acid. The content of ursolic acid in the ethanol extract of Eriobotrya was determined by Zuo et al. [13] using HPLC with Methanol: water (88:12) as the mobile phase and λ=210nm wavelength with Symmetry shield RP C-18 column.
Zhao et al. [14] reported RP-HPLC method for simultaneous determination of betulin and betulinic acid in white birch bark using Diamonsil C18 column and acetonitrile:water (88:16, v/v) as mobile phase and UV detector of λ=210nm wavelength. Quantification of betulinic acid in leaf extract of Orthosiphon stamineus was carried out by Akowuah et al. [15] using HPLC with C-18 column and acetonitrile: water acidified to pH 2.08 with phosphoric acid (80:20) at λ=210 nm wavelength. Puder et al. [16] developed process for extraction of betulinic acid from ground plane bark and used HPLC and NMR for determination and quantification of betulinic acid. Markus [17] investigated process for obtaining betulinic acid from methanolic extract of ground plane tree cortex and used HPLC for quantification of betulinic acid. HPLC method was used by Abreu et al. [18] for determination of betulinic acid from extract of Hypericum brasiliense with C18 microsorb- MV column with acetonitrile: water (9:1) pH 3.0 adjusted with phosphoric acid at λ=210 nm wavelength.
With the above discussion and from the literature available, number of medicinal plants consists of ursolic acid and betulinic acid, which can be extracted simultaneously due to their wide medicinal applications. All the existing methods separately determine either ursolic or betulinic acid from their extracts and each of them was tried to apply for a mixture of both these acids and it was found that none of them is suitable to apply for simultaneous determination of ursolic acid or betulinic acids from their mixtures.
To the best of our knowledge there is no report available that separates and estimates both these acids from their mixtures. The objective of this work is to determine the ursolic acid and betulinic acid content simultaneously by using one method from their methanol extract of Vitex Negundo Linn leaves, because none of the earlier method really does it.
Materials and Methods
The leaves of VN were collected from nearby area of Raigad district, Maharashtra, India. The leaves were washed with water and shade dried (at 30°C for 168 hrs) and pulverized. The powdered mass was then sorted into various sizes by screening technique and 0.42-0.60 mm size was taken for further experiments.
Ursolic acid (90%) and betulinic acid (90%) standard were obtained from Fluka, USA and used as standards for calibration of the HPLC system. Acetonitrile and methanol of HPLC grade were obtained from S.D. Fine, India.
Apparatus and chromatographic conditions
Standards of ursolic acid and betulinic acid dissolved in methanol and all unknown samples of methanolic extract were analyzed by HPLC system of Waters’ (USA). The system consists of Waters HPLC 600 pump, a Rheodyne 7725i injector with 5 μl sample loop, on-line degasser AF (Waters, USA), Waters 2487 Dual λ absorbance UV detector (at λ=210 nm, for ursolic acid and betulinic acid) with 0.05 aufs sensitivity. Ursolic acid and betulinic acid were analyzed using a Symmetry®
C-18 (4.6 × 250 mm, 5 μm) column equipped with automatic temperature (± 0.1°C) controller module. An isocratic mobile phase of acetonitrile: methanol (80:20 v/v) with an elution volume of 0.5 ml/min was selected for identification of both acids. The column temperature was maintained at 35°C ( ± 0.1°C).
Preparation and analysis of extract of VN leaves
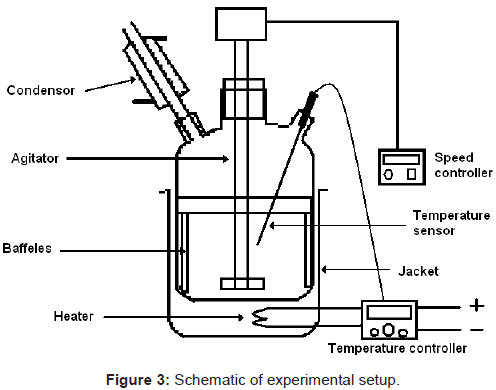
5 gm of shed dry and crashed leaves (particle size 0.42 to 0.60 mm) was subjected to fully baffled 150 ml stirred borosilicate glass vessel (5 cm ID, and 9.5 cm height) along with methanol (100 ml) as solvent. A four blade turbine type agitator (2 cm diameter) was used for stirring at 500 rpm to ensure all particles remain in suspended condition. Extraction of ursolic and betulinic acids were carried out at 28°C ( ± 1°C) for 2 hrs. The schematic experimental setup is as shown in Figure 3. The samples were collected at fixed interval of time. The collected samples were filtered through 0.2 μm membrane filter and analyzed in HPLC system for determination of recoverable ursolic acid and betulinic acid from their mixtures.
LC-MS analysis of ursolic acid and betulinic acid standard and methanolic extract of Vitex Negundo Linn leaves
The methanolic solutions of standards of ursolic acid and betulinic acid were analyzed with LC-MS for confirmation of the peaks and its positions. The methanolic extract of Vitex Negundo linn leaves also analyzed with LC-MS for identification of ursolic acid and betulinic acid in extract. Waters Aquity LC-MS system, Waters TQD Mass detector and Waters Masslynx 4.1 software was used along with Symmetry® C-18 column (4.6 × 250 mm, 5 μm) with same mobile phase mentioned earlier.
Results and Discussion
Determination of ursolic acid and betulinic acid from methanolic extract
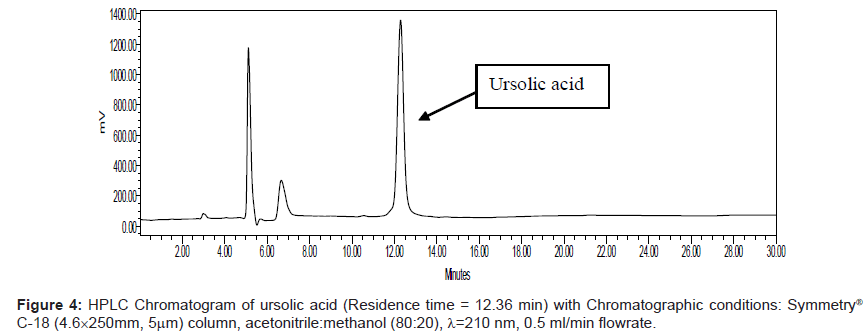
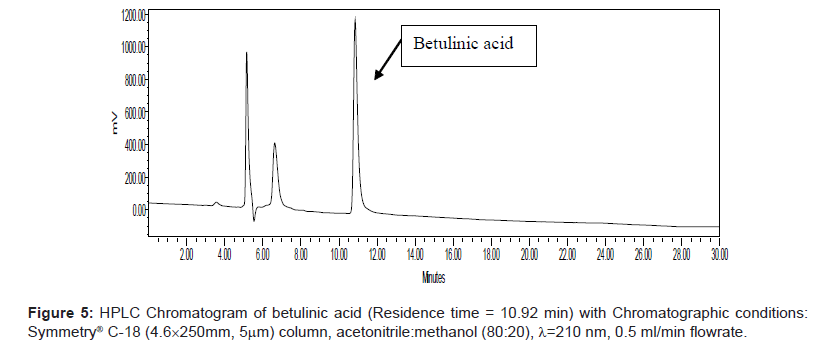
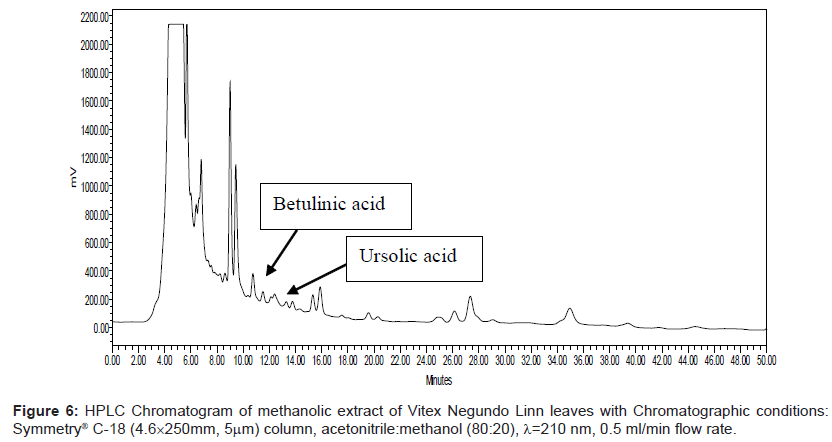
Series of HPLC methods available in literature were tried to separate ursolic acid and betulinic acid content from the methanolic extract, but none of them was found suitable for separation of both acids by any single method. To overcome this problem, we tried different possibilities of solvent compositions to change the polarity of mobile phase for ursolic acid and betulinic acid separation from the methanol extract. The reported HPLC method was found suitable for ursolic acid and betulinic acid separation and quantification simultaneously. In this method, acetonitrile:methanol (80:20) was used as mobile phase with 0.5 ml/min elution flow rate, λ=210 nm UV wavelength with 0.05 aufs sensitivity. Symmetry® C-18 (4.6 × 250 mm, 5 μm) column was used for separation and isolation of ursolic and betulinic acids. The HPLC chromatograms of ursolic and betulinic acid using the developed method are shown in Figure 4 and Figure 5.
The retention time for ursolic acid is 12.36 min ( ± 0.2 min) while for betulinic acid is 10.92 min ( ± 0.2 min). The method developed certainly indicates improved resolution of peaks and better selectivity. The chromatogram for methanol extract of leaves is shown in Figure 6. A distinct peak appearing at 12.36 min indicates ursolic acid while the peak at 10.92 min matches with betulinic acid retention time. The peak fractions appearing at 12.36 mins and 10.92 mins were separately collected in separate sample vowels after injecting the extracts several times. These two fractions were then purified and MS analysis was carried out to confirm the collected fractions are separately of ursolic and betulinic acids respectively.
Ursolic acid calibration
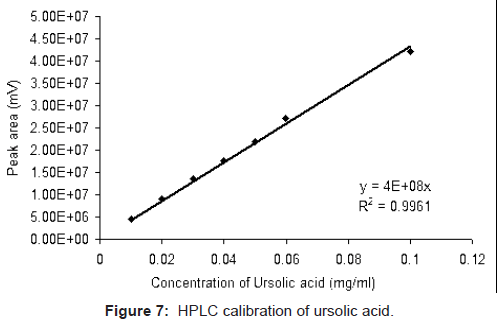
Standard solutions of ursolic acid (0.01, 0.02, 0.03, 0.04 0.05 0.06 and 0.1 mg/ml) were prepared in methanol. The solutions were filtered through 0.2 μm (PALL Ultipor N66) membrane filter and analyzed in HPLC. Evaluation of each point was repeated thrice. The area under peak of ursolic acid vs. concentration plot shows a linear fit with correlation coefficient of 0.9961 (Figure 7). The same calibration chart was subsequently used for quantification of unknown samples.
Betulinic acid calibration with HPLC
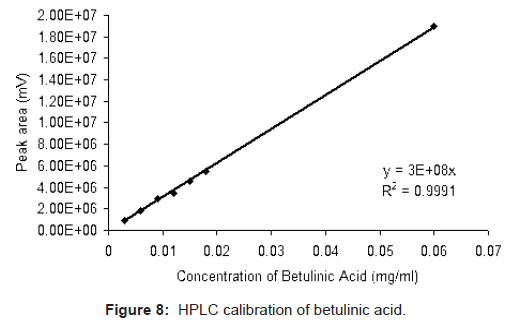
Standard solutions of betulinic acid (0.003, 0.006, 0.009, 0.012, 0.015, 0.018, and 0.06 mg/ml) were prepared in methanol. All standard as well as samples were filtered through 0.2 μm (PALL Ultipor N66) membrane filter and injected in the HPLC system through another syringe filter 0.2 μm. The area under the peak of betulinic acid was calculated using Waters Empower-II software and that corresponds to the concentration of betulinic acid present. The plot of area against concentration of betulinic acid standard injected shows a linear fit with correlation coefficient 0.9991 (Figure 8). The same calibration chart was subsequently used for quantification of unknown samples.
LC-MS analysis of ursolic acid and betulinic acid standard and methanolic extract of Vitex Negundo Linn leaves
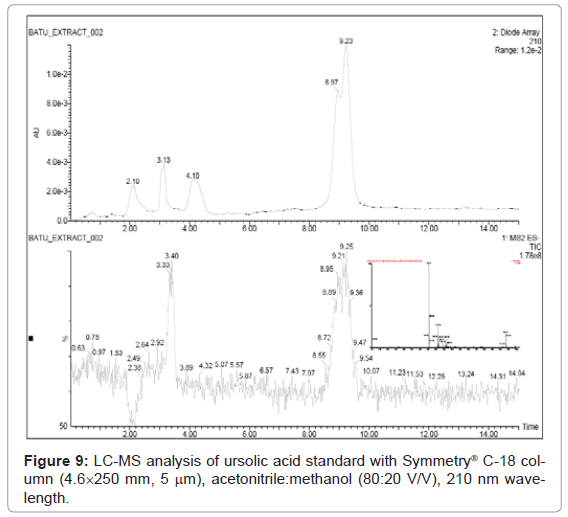
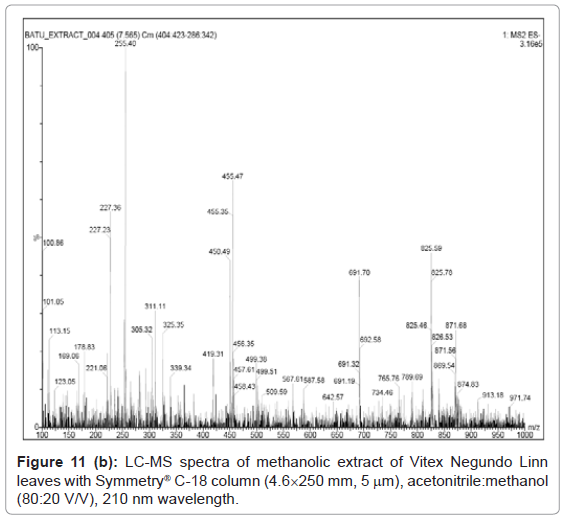
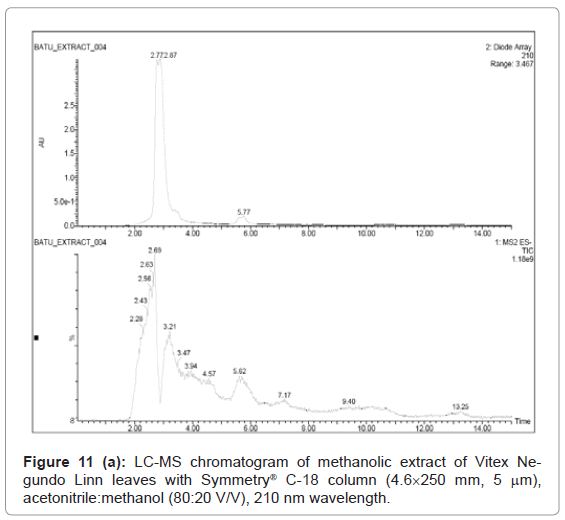
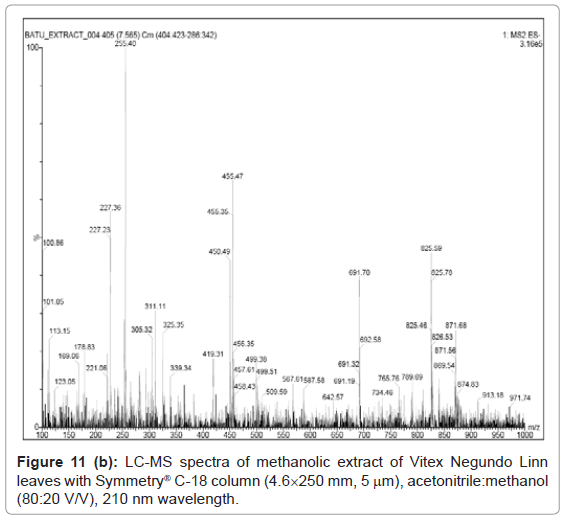
Figure 9, Figure 10 and Figure 11 shows the chromatogram and spectra of the ursolic acid, betulinic acid and methanolic extract of Vitex Negundo Linn leaves respectively by LC-MS. The top chromatograms in each figure shows that the position of ursolic acid and betulinic acid at 210 nm wavelength of UV Detector and the bottom chromatograms shows that the position of ursolic acid and betulinic acid with MS detector and spectra shows the mass of that peak fraction which matches with standards of ursolic acid and betulinic acid. These chromatograms are matching with each other at that position and show the exact mass of pure compounds (ursolic acid and betulinic acid). Figure 11 (a) represents the chromatograms with UV and MS detector while Figure 11 (b) represents the spectra of extracted sample of identified peaks of ursolic acid and betulinic acid. From these figures it is clear that the HPLC method used for analysis of ursolic acid and betulinic acid is valid. The results obtained by UV detector at 210 nm wavelength are matching with MS detector for analysis of ursolic acid and betulinic acid from methanolic extract of Vitex Negundo Linn Leaves.
Method validation
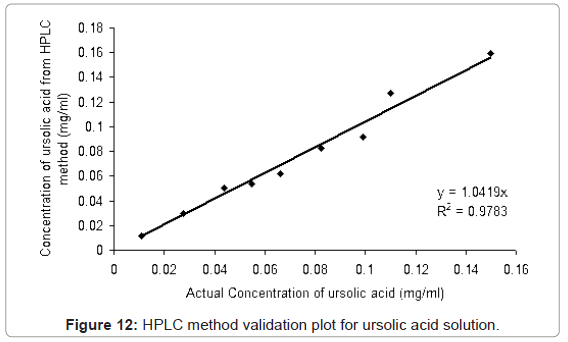
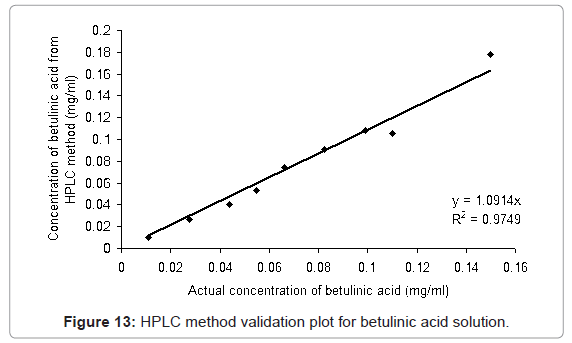
The HPLC method developed was validated with different proportions of ursolic acid and betulinic acid standards (ursolic acid: betulinic acid; 0:100, 10:90, 25:75, 40:60, 50:50, 60:40, 75:25, 90:10, 100:0 (v/v)) solutions in methanol. From Figure 12 and Figure 13, it is observed that the method is linear for the ursolic acid and betulinic acid concentration for the range 0.099 to 0.10 mg/ml concentration. Above 0.1 mg/ml concentration of both acids the linearity lost. The validation plot shows the R2 value more than 0.97 for both acids (Figure 12 and Figure 13). This value of standard deviation and the linearity equation shows the method is valid for the given range of concentrations.
Batch extraction of ursolic acid and betulinic acid from leaves
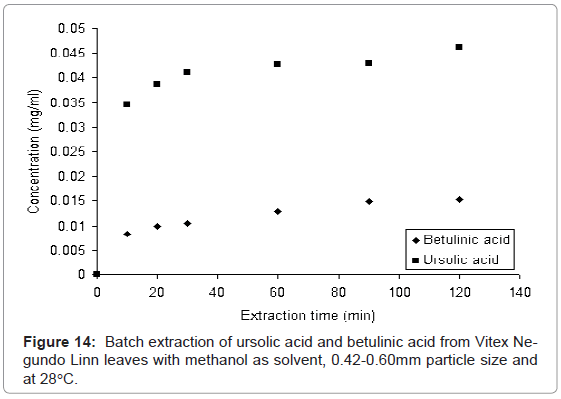
The batch extraction experiment was carried out with methanol as solvent and at 28°C to extract the ursolic acid and betulinic acid from leaves of Vitex Negundo Linn. The collected samples were analyzed with proposed HPLC method to determine the ursolic acid and betulinic acid content. Figure 14 shows, how the concentration of ursolic acid and betulinic acid increases with increase in extraction time. This increase in concentration of both acids is due to diffusion mechanism of solutes (ursolic acid and betulinic acid) in solvent (methanol).
This experiment was carried out to verify the validity of the proposed method. It is found that the proposed HPLC method is valid for simultaneous determination of ursolic acid and betulinic acid from leaves of Vitex Negundo Linn.
Conclusion
The HPLC methods available in the literature are tried with the given conditions and with Symmetry C-18 column. These methods did not show any results in case of ursolic acid and betulinic acid for extract of Vitex Negundo Linn. The developed method of HPLC is suitable for simultaneous determination of ursolic acid and betulinic acid from leaves of Vitex Negundo Linn. The method showed linearity for ursolic acid and betulinic acid different concentrations. LC-MS analysis shows the validation of method. From the results of batch extraction experiments, the method is accurate, sensitive and has a good reproducibility.
Acknowledgements
The financial support from AICTE Project (Grant no. 8023/RTD/RPS52/2004- 05) and TEQIP (World-Bank project) for this research work is gratefully acknowledged. Authors are thankful to Waters India Ltd for providing LC-MS facility.
References
- Chadha YR (1976) The wealth of India: A dictionary of Indian raw material and industrial products. CSIR (Council of Scientific & Industrial Research), New Delhi, India.
- Taneja SC, Gupta RK, Dhar KL, Atal CK (1979) The essential oil of Vitex negundo linn. Indian Perfumer, India.
- Dutta KP, Chowdary S, Chakravarty AK, Achari B, Pakrashi CS (1993) Studies of Indian medicinal plants part LXXV; Nishindaside, a novel iridoid glycoside from Vitex negundo. Tetrahadran 39: 3067-3072.
- Prema MS, Misra GS (1978) Leucoanthocynidins of Vitex Negundo. Ind J Chem, India.
- Chawla AS, Sharma AK, Ha SS, Dhar KL (1992) Chemical investigations and anti-inflammatory activity of Vitex negundo seeds. J Nat Prod 55: 163-167.
- Liu J (1995) Pharmacology of oleanolic and ursolic acid. J Ethnopharmacol 49: 57-68.
- Chandramu C, Manohar RD, Krupadanam DG, Dashavantha RV (2003) Isolation, characterization and biological activity of betulinic acid and ursolic acid from Vitex Negundo L. Phytother Res 17: 129-134.
- Pisha E, Chai H, Lee IS, Chagwedera TE, Farnsworth NR, et al. (1995) Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat Med 10: 1046-1051.
- Simone CB, Eloir PS, Valquiria LB (2005) HPLC method to assay total saponins in Ilex paraguariensis aqueous extract. J Braz Chem Soc 16: 723-726.
- Liao Q, Yang W, Jia Y, Chen X, Gao Q, et al. (2005) LC-MS determination and pharmacokinetic studies of ursolic acid in rat plasma after administration of the traditional chinese medicinal preparation Lu-Ying extract. Yakugaku Zasshi 125: 509-515.
- Chen JH, Xia ZH, Tan RX (2003) High perfrmance liquid chromatographic analysis of bioactive triterpenes in Perilla frutescens. J Pharm Biomed Ana 32: 1175-1179.
- Novotny L, Abdel-Hamid M, Hamza H, Masterova I, Grancai D (2003) Development of LC-MS method for determination of ursolic acid: application to the analysis of ursolic acid in Staphylea holocarpa Hemsl. J Pharm Biomed Anal 31: 961-968.
- Zou S, Chen W, Li K (2004) Preparation and HPLC analysis of ursolic acid from Eriobotrya japonica Lindi. Acta Agri Univer Jiangxiensis 26: 808-810.
- Zhao G, Yan W, Cao D (2007) Simultaneous determination of betulin and betulinic acid in white birch bark using RP-HPLC. J Pharm Biomed Anal 43: 959-962.
- Akowuah GA, Zhari I, Norhayati I, Sadikun A, Sundram K, et al. (2003) Quantification of betulinic acid in leaf extracts. J Tropical Medicinal Plants 4: 225-228.
- Puder CH, Graef H, Thumerer MJ, Heitzmann M (2007) Process for the extraction of betulinic acid. United State Patent, USA.
- Markus S (2005) Process for obtaining betulinic acid. United State Patent, USA.
- Abreu AN, Porto ALM, Marsaioli AJ, Mazzafera P (2004) Distribution of bioactive substances from Hypericum brasiliense during plant growth. Plant Science 167: 949-954.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 29031
- [From(publication date):
July-2012 - Dec 20, 2025] - Breakdown by view type
- HTML page views : 23011
- PDF downloads : 6020