Research Article Open Access
A Hospital Based Cohort Study of Colorectal Cancer Cases Treated at Braga Hospital, Northern Portugal
Sandra F Martins1-3, Ricardo Amorim1,2, Rui M Reis1,2, Céline Pinheiro1,2, Mesquita Rodrigues4, Fátima Baltazar1,2 and Adhemar Longatto Filho1,2,5*1Life and Health Sciences Research Institute (ICVS), School of Health Sciences, University of Minho, Braga, Portugal
2ICVS/3B’s-PT Government Associate Laboratory, Braga/Guimarães, Portugal
3Surgery Department - Centro Hospitalar Trás-os-Montes e Alto Douro, Portugal
4Coloproctology Unit–Hospital Braga, Portugal
5Laboratory of Medical Investigation (LIM) 14, Faculty of Medicine, University of São Paulo, Brazil
- *Corresponding Author:
- Adhemar Longatto Filho
Life and Health Sciences Research Institute (ICVS)
School of Health Sciences, University of Minho, Braga, Portugal
Tel: + 351 253 604827
Fax: + 351 253 6048472
E-mail: longatto@ecsaude.uminho.pt
Received date: September 04, 2013; Accepted date: October 23, 2013; Published date: October 30, 2013
Citation: Martins SF, Amorim R, Reis RM, Pinheiro C, Rodrigues M, et al. (2013) A Hospital Based Cohort Study of Colorectal Cancer Cases Treated at Braga Hospital, Northern Portugal. J Gastroint Dig Syst 3:146. doi: 10.4172/2161-069X.1000146
Copyright: © 2013 Martins SF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Background: Colorectal cancer (CRC) is the third most common cancer and the fourth most frequent cause of cancer death worldwide. Nonetheless, despite being a frequent cancer on which many epidemiological international studies have already been written, Portuguese epidemiological data are scarce and in particular there are very few specific data for Minho Region, which is traditionally recognized as a high incidence area.
Aim: Characterize CRC patients treated at Braga Hospital.
Methods: Data regarding clinical and preoperative diagnostic examinations, operative reports and histopathological and follow-up data was collected prospectively and stored in two Excel PC databases (colon and rectal cancer) and statistically analysed using the Statistical Package for the Social Sciences, version 19.0 (SPSS Inc., Chicago, Illinois, USA). All comparisons were examined for statistical significance using Pearson’s chi-square (χ2) test and Fisher’s exact test (when n<5), with the threshold for significance P values <0.05. Overall survival and Survival free disease were both assessed using the Kaplan-Meier method.
Results: The study comprises 672 patients with histological diagnosis of CRC treated in Braga Hospital between 2005 and 2009. It included 62.3% males and 37.7% females and most patients (60.5%) were between 61-80 years old. 65.3% of the cases arose from colon cancer and 34.7% from rectal cancer. We observed that 94.8% of the patients had no previous history of colorectal polyps. 4.1% had a previous personal history of CRC and 7.7% of a different cancer. 9.7% had a positive CRC family history. Most patients (81.3%) were symptomatic at diagnosis, while 18.8% were detected by routine colonoscopies. Colon and rectal cancer from most patients was at IIA stage and IV stage respectively. Follow-up time ranged between 1 and 5 years and, during this period, 26.7% of colon cancer patients and 25.3% of rectal cancer patients died from a colorectal cancer-related cause; also, 14.6% and 19.3% respectively had recurrence, mainly in the liver.
Conclusion: This is the first study of a large cohort of CRC patients from the Minho Region in Northern Portugal. The large majority of the 672 cases were diagnosed because symptomatic and at an advanced stage, with a relatively poor prognosis. These findings emphasize the need to start a screening program and diagnose CRC at an early stage, thus increasing cure rates and improving resource management.
Keywords
Colorectal cancer; Epidemiological data
Abbreviations
CRC: Colorectal Cancer; WHO: World Health Organization; RORENO: North Regional Oncologic Registry; MR: Magnetic Resonance; EUS: Rectal Endoscopic Ultrasound; OS: Overall Survival; DFS: Survival Free Disease
Background
Colorectal cancer (CRC) is the third most common cancer [1-3] and the fourth most frequent cause of cancer deaths [1-4]. Globally, CCR incidence varies widely, with higher rates in North America, Australia and Western Europe, and the lowest rates in developing countries [5], although, in recent years, increasing colorectal cancer rates have been reported in these countries [6]. According to the World Health Organization (WHO), CRC is one of the most prevalent diseases of the Western world [7] and the second most common cause of death from malignant disease in the Western countries [8,9].
In Europe, data from WHO and National Registries reveals that CRC is the second most common cancer, after lung cancer in males and breast cancer in females [10]. European countries rank highest in the global statistics, both in terms of incidence and mortality [10,11] but, over the past twenty-five years, mortality rates among Caucasians have steadily declined [12] and data from WHO between 1997 and 2007 shows a decline in CRC mortality from 19.7 to 17.4/100,000 in men and 12.5 to 10.5/100,000 in women [13]. This recent decrease in CRC mortality rates in several European countries is likely due to improvements in early diagnosis and treatment, with a consequent higher survival from the disease [13].
In Portugal, data from the National Statistic Registry revealed that CRC is the second most common cancer, after lung cancer, with an incidence of 5000/year and one of the main causes of death by neoplastic disease [14]. In the North of Portugal, data from RORENO (North Regional Oncologic Registry) shows that, in 2005, CRC was the second most prevalent cancer, followed by prostate cancer in males and breast cancer in females [15], and the third cause of death by neoplastic disease in men, preceded by lung and gastric cancer, and the second cause of death by neoplastic disease in women, after breast cancer [16].
Most CRC are sporadic cancers, without a colorectal cancer family history. The remaining arise in individuals with an inherited predisposition to the disease (at least 15%) and in individuals with welldefined mendelian syndromes (almost 5%); in this setting, colorectal cancer risk is very high [14,17]. Since epidemiological data on CRC is scarce in Portugal, and no data exists about the Minho region, the authors carried out a descriptive study to characterize CRC patients treated at Braga Hospital, a city in the North of Portugal with an area of reference of 300,000 patients.
Methods
Data from 672 patients with CRC diagnosis treated in Braga, Northern Portugal, between January 1st 2005 and January 1st 2010 was used to describe the distribution of colorectal carcinoma. The data was collected prospectively and includes: clinical and preoperative diagnostic examinations, operative reports by the surgeons, histopathological and follow-up data. This data was organized in two Excel databases (colon and rectal cancer).
Clinical and preoperative diagnostic examinations included: age, gender, clinical presentation, past oncologic history, tumour localization, histological type, macroscopic appearance and preoperative staging.
Tumour localization was recorded and classified as right sided (caecum, ascending colon, hepatic flexure and transverse colon) or left sided (splenic flexure, descending colon, sigmoid colon) and rectum (between anal verge and 15 cm at rigid rectoscopy). Rectal cancer localization was subdivided as superior, middle and lower third (≤ 15 and >10 cm; ≤ 10 and >5 cm and ≤ 5 cm from anal verge, respectively).
Operative reports by surgeons, including presence of perforation, tumour mobility and type of surgery, were also collected. All patients received antibiotic and thrombosis prophylaxis and all operations were performed by or under supervision of senior surgeons. An emergency surgery was defined as a surgery performed for obstruction or perforation of the colon or rectum. The histopathological reports included: tumour extent (T), extent of spread to lymph nodes (N), presence of distant metastasis (M), tumour differentiation, resection margin involvement and lymphatic and blood vessel invasion. The level of positive lymph nodes was not described in all specimens. The histological type of CRC was determined by two experienced pathologists and tumour staging was graded according to TNM classification, sixth edition.
All patients were followed up periodically and their outcomes were investigated and collected until July 2012.
Ethics committee approval
The study protocol was approved by the Ethics Committee of Braga Hospital. All patients provided written consent.
Statistical analysis
All clinical, surgical and follow-up data was collected and stored in an Excel PC database and all data was analysed statistically using the Statistical Package for the Social Sciences, version 19.0 (SPSS Inc., Chicago, Illinois, USA).
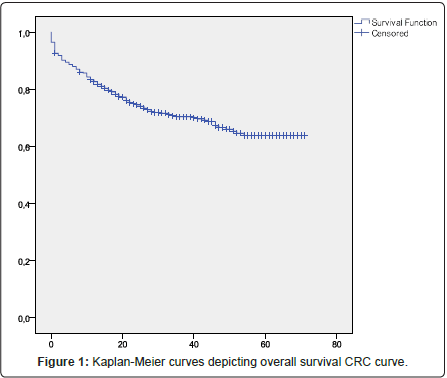
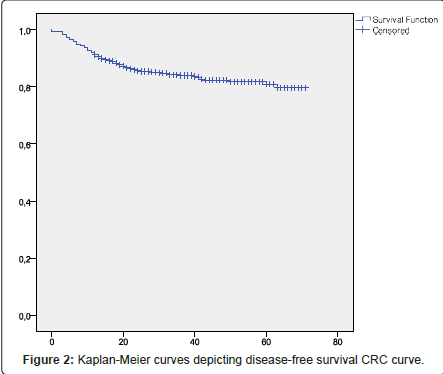
Overall survival (OS) was defined as time from disease diagnosis until death from any cause and Survival free disease (DFS) was defined as time from disease diagnosis until disease relapse; both were assessed using the Kaplan-Meier method.
Results
Patient history data
The casuistic included 672 patients, 419 (62.4%) males and 253 (37.6%) females; the age range of most patients (61%) was 61-80 years old, 20.4% (n=137) was 41-60 years old; 16.1% (n=108) was older than 81 and 2.5% (n=17) was younger than 40 years old. Except for the group older than 81 years old, CRC incidence was more frequent in men (Table 1). 94.8% (n=637) of the patients had no history of previous colorectal polyps; from the patients with polyps, 4.3% (n=29) were tubular, 0.4% (n=3) adenomatous, 0.3% (n=2) tubulovillous and 1 was non-classified. From overall patients, 4.1% (n=28) had a previous personal history of CRC and 7.7 % (n=52) had personal history of other cancer. 9.7% (n=65) had a positive CRC familial history.
| Age | Males ( %) | Females ( %) | Total (%) |
| 0 - 40 | 9 | 8 | 17 (2.5) |
| 41 -60 | 89 | 48 | 137 (20.4) |
| 61 - 80 | 267 | 143 | 410 (61) |
| 81+ | 54 | 54 | 108 (16.1) |
Table 1: Age and sex distribution of Colon and Rectal Cancer Cases.
Most of patients, 81.3% (n=546), with CRC were symptomatic at diagnosis, the remainder 18.8% (n=126) were asymptomatic and detected by routine colonoscopies. Of the symptomatic patients, 82.11% (n=450) presented symptoms 6 months prior to colonoscopy and 14.6% (n=98) after 6 months. Among the 672 patients, 439 tumours (65.3%) arouse from colon and 233 (34.7%) from rectum.
Colon cancer
Symptoms: In symptomatic patients, 17.1% (n=75) presented digestive bleeding, followed by large bowel obstruction, 15% (n= 66). Other frequent symptoms observed were: change in bowel habits (8.9%), change in bowel habits with digestive bleeding (8.6%), constitutional symptoms (6.6%), change in bowel habits with abdominal pain (6.4%) and abdominal pain (4.8%) (Table 2).
| Symptom | n (%) |
| Digestive bleeding | 75 (17.1) |
| Large bowel obstruction | 66 (15.0) |
| Change in bowel habit | 39 (8.9) |
| Digestive bleeding + changes in bowel habit | 38 (8.6) |
| Constitutional symptoms | 29 (6.6) |
| Abdominal pain + changes in bowel habit | 28 (6.4) |
| Study (ascites; anemia, deep venous thrombosis, hepatic metastasis; occult blood losses, colonvesical fistula) | 23 (5.2) |
| Abdominal pain | 21 (4.8) |
| Parcial large bowel obtruction | 13 (3.0) |
| Large bowel perfuration | 8 (1.8) |
Table 2: Summary of presentation symptoms and signs of Colon Cancer Cases.
Localization: Most cancers were left-colon, 56.7% (n=249), mainly in the sigmoid colon.. Right-sided tumours comprised 27% (n=119) of the patients (Table 3).
| Characteristics | Colon n (%) | Rectum n (%) |
| Localization | Caecum: 34 (7.7) Ascending colon: 33 (7.5) Hepatic flexure: 52 (11.8) Transverse colon: 20 (4.6) Splenic flexure: 28 (6.4) Descending colon: 16 (3.6) Sigmoid colon: 193 (44) Rectosigmoid transition: 12 (2.7) |
Proximal: 49 (21) Middle: 118 (50.6) Distal: 66 (28.3) |
| Macroscopic appearance | ||
| Polypoid/vegetant ulcerated Infiltrative Exofitic Villous No information |
207 (47.2) 92 (21.0) 37 (8.4) 49 (11.2) 0 (0) 54 (12.3) |
130 (55.8) 49 (21.0) 25 (10.7) 21 (9.0) 1 (0.4) 7 (3) |
| Histological Staging Stage: | ||
| 0 I IIA IIB IIIA IIIB IIIC IV |
9 (2.1) 55 (13.0) 142 (33.7) 11 (2.6) 6 (1.4) 95 (22.5) 18 (4.3) 79 (18.7) |
21 (10.3) 38 (18.7) 43 (21.2) 0 (0.0) 12 (5.9) 31 (15.3) 13 (6.4) 37 (18.2) |
| Serosal involvement | ||
| With Without No information |
295 (69.9) 103 (24.4) 24 (5.7) |
109 (53.7) 70 (34.5) 24 (11.8) |
| Tumour differentiation | ||
Table 3: Summary of Clinical and Histopathological characteristic of Colon and Rectal Cancer Cases.
Diagnosis and staging: Diagnosis was made by total colonoscopy in 76.1% (n=334) of cases and rectosigmoidoscopy in 13% (n=57). In 10.9% (n=48), diagnosis was made by other imagiological exams and patients did not have a preoperative colonoscopy.
Most lesions (47.2%, n=207) were polypoid/vegetant cancers (Table 3). In 19.1% (n=84) of patients, synchronous lesions were observed. Pre-operative colon biopsy revealed colon adenocarcinoma in 83.8% (n=368) of the patients; 3.9% (n=17) had dysplastic lesions, 0.4% (n=2) had mucinous adenocarcinoma and for the remainder 11.9% we did not have pre-operative information. 85.7% (n=376) of patients were staged by computerized axial tomography and most patients (79.1%; n=347) with colon cancer had a localized cancer at diagnosis. Most patients with disseminated disease had hepatic metastasis, followed by lymph node metastasis.
Histological staging: Histological staging was determined by two experienced pathologists and tumour staging was graded according to TNM classification, sixth edition (American Joint Committee on Cancer). In the majority of patients (33.7%; n=142) colon cancer was stage IIA, followed by stage IIIB (22.5%; n=95) (Table 3).
Macroscopic serosal involvement: Of the patients examined, 295 (69.9%) presented macroscopic serosal involvement and 103 (24.4%) presented no macroscopic serosal involvement (Table 3).
Tumour differentiation: Most patients, 172 (40.8%), presented a moderated-differentiated tumour, followed by well and poorlydifferentiated tumour (168 and 41 patients, respectively). 0.2% of the patients (1 patient) presented an undifferentiated tumour Vascular Invasion (Table 3). Although no specific marker of lymphatic or hematogenous vessels was used, we documented that 229 patients (54.2%) had venous vessel invasion and 166 (39.3%) had lymphatic vessels invasion. In 156 (36.9%) and 209 (49.5%) patients, respectively, no invasion was documented and for the remainder no information was mentioned (Table 3).
Follow-up: A total of 137 patients (31.2%) died from all causes, 27.8% (122 patients) had a colorectal cancer-related cause and the remaining 3.4% (15 patients) died in the post-operative period (mortality within 30 days of surgery). Follow-up time ranged between 2 and 7 years; 14.6% (62 patients) had recurrence during followup. Stage IIIB was the stage most frequently associated with tumour recurrence. Most metastasis occurred in liver, followed by lymph node and lung. Local recurrence occurred in nine cases. Most metastasis and recurrence was asymptomatic (79.0%; n=49), and 29.0% of them (n=18) presented an asymptomatic elevation of tumour markers. The remaining cases presented patients with abdominal pain (4.8%; n=3), abdominal mass (4.8%; n=3), intestinal obstruction (3.2%; n=2), bone pain (3.2%; n=2), supraclavicular mass (1.6%; n=1), enterocutaneous fistula (1.6%; n=1) and pathological fracture (1.6%; n=1).
Rectal cancer
Symptoms: Most rectal cancer patients (88.5%, n=206 patients) were symptomatic at diagnosis. 20% (n=47) presented digestive bleeding, followed by digestive bleeding with change in bowel habits, 17.4% (n=41). Other frequent symptoms observed were: change in bowel habits (13.2%; n=31) and large bowel obstruction (4.7%; n=11) (Table 4).
| Symptom | n (%) |
| Digestive bleeding | 47 (20) |
| Digestive bleeding + change in bowel habit | 41 (17.4) |
| Change in bowel habit | 31 (13.2) |
| Large bowel obstruction | 11 (4.7) |
| Incomplete stool evacuation sensation | 11 (4.7) |
| Tenesmus | 10 (4.2) |
| Tenesmus + Digestive bleeding | 10 (4.2) |
| Digestive bleeding + constitutional symptoms | 7 (3.0) |
| Abdominal pain | 7 (3.0) |
| Constitutional symptoms | 6 (2.6) |
| Abdominal pain + digestive bleeding | 5 (2.1) |
| Tenesmus + changes in bowel habit + Digestive bleeding | 5 (2.1) |
| Study (hepatic metastasis, pelvic mass) | 4 (1.7) |
| Tenesmus + changes in bowel habit | 4 (1.7) |
| Change in bowel habit + constitutional symptoms | 3 (1.3) |
| Large bowel perfuration | 2 (0.9) |
| Urgency | 1 (0.4) |
| Anal pain | 1 (0.4) |
Table 4: Summary of presentation symptoms and signs of Rectal Cancer Cases.
Localization: Of the 233 rectal cancers, most (50.6%, n=118) were localized in the middle third (Table 3).
Diagnosis and staging: In rectal cancer patients, diagnosis was made by total colonoscopy in 79.8% (n=186) and rectosigmoidoscopy in 18.9% (n=44). In 1.3% of cases (n=3), it was impossible to perform an endoscopic exam (rectal stenosis). Most lesions (55.8%, n=130) were polypoid/vegetant cancers (Table 3). Synchronous lesions were observed in 10.3% (n=24) of the patients.
Pre-operative biopsy revealed rectal adenocarcinoma in 91.4% (n=213) of the patients, invasive adenocarcinoma in 2.1% (n=5), adenomatous dysplastic lesions in 4.7% (n=11); villous lesions in 1.3% (n=3) and mucinous adenocarcinoma in one patient (0.4%). Of the 233 patients, 27.0% (n=63) had synchronic metastasis at diagnosis, more frequently lymph node and hepatic metastasis. Pelvic magnetic resonance (MR) and rectal endoscopic ultrasound (EUS) were used in combination for local staging. After staging, 26% (61 patients) had indication for neoadjuvant therapy, 21% (49 patients) underwent chemotherapy and radiotherapy, and the remaining had not done neoadjuvant therapy due to comorbidities (2 patients) or underwent chemotherapy or radiotherapy alone due to specific contraindications.
Histological staging: Post-operative histological staging was determined by two experienced pathologists and tumour staging was graded according to the TNM classification, sixth edition (American Joint Committee on Cancer). Most patients with rectal cancer were stage IIA (21.2%) and stage I (18.7%), followed by stage IV (18.2% patients). In 8 patients, post-operative histological stage was not determinate because the patients underwent surgery without resection (ex. derivative colostomy) (Table 3).
Macroscopic serosal involvement: Of the patients examined, 109 (53.7%) presented macroscopic serosal involvement and 70 (34.5%) presented no macroscopic serosal involvement (Table 3).
Tumour differentiation: Most patients, 80 (39.4%), presented a moderated-differentiated tumour, followed by well and poorlydifferentiated tumour 73 (36.0%) and 9 (4.4%) patients, respectively). 1.0% of the patients (2 patients) presented an undifferentiated tumour; for 40 patients, this data was not mentioned (Table 3).
Vascular invasion: As previous mentioned, although no specific marker of lymphatic or hematogenous vessels was used, we documented that 113 (55.6%) patients had venous vessel invasion and 90 (44.3%) had lymphatic vessels invasion. In 59 (29.0%) and 81 (39.9%) patients, respectively, no invasion was documented and for the remainder no information was mentioned (Table 3).
Follow-up: A total of 52 patients (22.3%) died from all causes, 28.0% (42 patients) had a colorectal cancer-related cause and the remaining 4.3% (10 patients) died in the post-operative period (mortality within 30 days of surgery). Follow-up time ranged from 2 to 7 years; 18.0% (42 patients) had recurrence during follow-up. Stage IV was the stage most often associated with tumour recurrence/progression. Most metastasis occurred in liver, followed by lymph node and lung. Local recurrence occurred in nine patients. Most patients with metastasis and recurrences (73.8%; n=31) were asymptomatic and 14.2% of them (n=6) presented an asymptomatic elevation of tumour markers. In the case of symptomatic patients, the most frequent symptoms were a rectal mass (9.5%; n=4) and intestinal obstruction (4.7%; (n=2)).
CRC overall survival
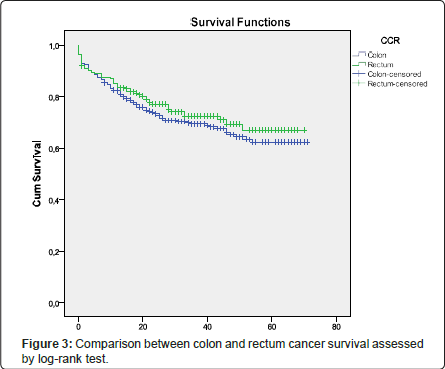
Overall survival (OS) was defined as time from disease diagnosis until death from any cause and Survival free disease (DFS) was defined as time from disease diagnosis until disease relapse; both were assessed using the Kaplan-Meier method (Figures 1 and 2). When the patients were divided into two groups by location-colon and rectum-no significant difference was found between the survival rates of the colon cancer group and rectal cancer group; this was assessed using the logrank test (Figure 3).
Discussion
CRC epidemiological data abounds in the worldwide literature, but in the case of the Portuguese population, this data is scarce and the existing studies are retrospective studies based in cancer registries but with little data that allows the characterization of the affected population. In the developed world, CRC represents a major public health problem [18]. In Portugal, it is the second most frequent cancer and the second cause of death by cancer [14,16] and, although CRC epidemiological data abounds in the worldwide literature, this data is scarce for the Portuguese population. The North of Portugal is traditionally considered to be an area of high CRC incidence. Braga Hospital, in the North of Portugal, has a reference area of 300,000 patients, but no epidemiological data exists in literature. Thus, we designed this prospective study to characterize the patients treated at this hospital, comparing with others of high incidence population studies.
Age and gender
In this study, most of the 672 patients (419, 62.4%) were male and the age range of most patients (61%) was 61-80 years old. Except for the group older than 81 years old, CRC incidence was more frequent in men. Similar results to the ones of the present study were found in literature and the Portuguese Cancer Registry, with CRC being more frequent at advanced age and in men. The last results about Portuguese population document that CRC is more frequent in men, although when observing separately colon and rectal cancer, we found that colon cancer is slightly more frequent in women (10.6% versus 10.4%) [15]. Similar results were observed in epidemiological Brazilian data [19,20]. Advanced age is the most significant risk factor for diagnosis of CRC, which is defined as a disease of elderly people, with the majority of cases arising after 65-70 years of age and with an incidence relatively lower under 40 years of age. Still, 15% of cases will occur in people ≤ 50 years old [1,3,10,11,21-26], although another study suggests a lower value (7%) [27] and a large study identifies it as one of the 10 most commonly diagnosed cancers among men and women aged 20-49 years [26]. Early onset of CRC is assumed to be indicative of genetic susceptibility [22], often associated with a positive family history [28]. In some studies, such younger patients presented more advanced disease and more aggressive tumour grades at diagnosis and had less favourable prognosis [26-29]. Prevalence of advanced colorectal neoplasms increases with age and is higher among men than women [18,25,29-31]. Cross-sectional analyses estimated that men reach an equivalent prevalence at a much younger age than women [30].
Anatomic distribution of tumours
Tumour distribution throughout colon and rectum depends on genetic and environmental factors involved in colorectal carcinogenesis and on the patient’s gender, race and age [10,29]. Several studies regarding CRC incidence have shown significant differences between colon and rectal cancer [10]. Some studies reveal that, in industrialized countries, CRC is more frequent in sigmoid colon and rectum [7,14]. Liu et al. reported a shift to the right colon in the high risk nations, for unknown reasons [32], and others have suggested that the frequency of right-sided colon cancer increases in elderly patients [10]. Our study revealed that colon cancer was more frequent than rectal cancer (65.3% versus 34.7%) and most colon cancers were left-sided (56.7% of all colon cancers). This data are in agreement with the world tendency and similar values (65.4%) were observed by Michelle Fraga Eisenhardt in Rio Grande do Sul (Brazil) [33]. In literature, studies have shown conflicting results when comparing prognosis and localization [34]. Reduced survival in left colon cancer compared to right colon was reported in a Norwegian study from 1987 and Aldrige et al. reported similar results [34-36], but no differences were detected in other studies [34,37-39]. In our study, the majority of colon cancer patients presented at diagnosis a localized cancer, stage IIA tumour; on the other hand, most rectal cancer patients were stage IV at diagnosis, supporting the results of the Norwegian study.
Past personal and family history
Epidemiological studies suggest that at least 15% of colorectal cancers arise in individuals with an inherited predisposition for the disease [14,17]. The literature also reveals that positive family history is strongly associated with CRC [10,25]. In our study, 94.8% of the patients had no history of previous colorectal polyps; 4.1% had a previous personal history of CRC; 7.7% had a personal history of other cancers and 9.7% had a positive family history for CRC. Knowing CRC natural history, we would expect a higher incidence of previous colorectal polyps history. This lower value seems to result from the low adherence of patients to perform colonoscopy without symptoms. Also, the values of family history are underestimated, since a significant number of patients do not know their relatives’ cause of death.
Symptoms
Symptoms of CRC can be nonspecific or quite fulminant [40]. Emergency situations are most commonly related to the complications of tumour obstruction [41] or tumour perforation [41,42]; these are relatively rare complications, both with a poor prognosis and high risk of recurrence [41]. In a meta-analysis, Jellema et al. analysed various symptoms of CRC and concluded that the symptoms most commonly investigated included abdominal pain, rectal bleeding, change in bowel habits, and perianal symptoms. Of the typical symptoms of CRC, only weight loss had some diagnostic value, with a fairly high specificity [25]. Most patients (81.3%) from our study were symptomatic at diagnosis. Analysing colon and rectal cancer, 77.4% (n=340 patients) and 88.5% (n=206 patients) were symptomatic at diagnosis, respectively. Digestive bleeding was the most frequent symptom at diagnosis for colon and rectal cancer (17.1% and 20%, respectively), followed by large bowel obstruction in colon cancer (15.0%) and digestive bleeding associated with change in bowel habits (17.4%) and change in bowel habits (13.2%) in rectal cancer.
Macroscopic serosal involvement
Macroscopic serosal involvement corresponds to a pT3 in TNM classification; In our series, 69.9% of colon cancers and 53.7% of rectal cancer presented macroscopic serosal involvement. When macroscopic serosal involvement is present, even in the absence of lymph node involvement (AJCC/UICC stage IIB classification), it also identifies high-risk disease requiring adjuvant therapy [43-45].
Tumour differentiation
Tumour differentiation is consistently recognized as an important prognostic parameter [41,46]. In our series, most of the cancers analysed were moderated-differentiated (40.8% for colon cancer and 39.4% for rectal cancer). Unlike some brasilian studies a higher value of well differentiated lesions were observed [33,47,48].
Vascular invasion
It was reported 54.2% and 55.6% of venous vessel invasion and 39.3% and 44.3 of lymphatic vessels invasion, for colon and rectal cancer respectively. CRC exploits the lymphatic and venous drainage of the intestinal wall for dissemination to regional lymph nodes and distant organs. Vascular invasion is an adverse prognostic factor in CRC, since it has been demonstrated repeatedly to be an independent prognostic factor [43,49,50]. The diagnosis of vascular invasion in CRC specimens may be exceedingly difficult with conventional haematoxylin-eosin staining alone [51]. Literature data reported a CRC vascular invasion in ranges from 10% to 89% [50], most likely due to the different criteria used for its identification or to patient selection. Suffice it to say that in some studies, for instance, no distinction was made between venous and lymphatic vessels intramural and extramural venous invasion, or colonic and rectal localization of the primary tumours [43].
Histological staging and follow-up
Stage at diagnosis plays a significant role in CRC survival [12,23-25,40,52-54] and is, in fact, the main prognostic factor in CRC [1-3,12,23,24], but it is difficult to accurately determine the stage prior to surgical treatment [55]. Staging has evolved over time and, currently, the TNM system is used. It is an evaluation system based on 3 variables: primary tumour (T), regional nodes (N) and metastasis (M) [40,52]. In the past, patients presenting the same stage of CRC were considered similar in terms of prognosis. The new staging criteria recognize that they are usually quite different and subsets of patients with varying survival statistics can be found [40,55]. Less than one quarter of patients’ present early disease [Stage I] that is curable by surgical resection [12,23,40] and more than 20% of CRC patients present stage IV disease at diagnosis [40]. This has an impact in five year survival rates and we can expect a five-year survival rate greater than 90% for stage I [12,23,25] and less than 10% for stage IV [25]. On the other hand, around 40% of the patients diagnosed with CRC eventually develop metastatic disease [24] and about two-thirds of the patients undergo resection with curative intent, but 50% of the patients still die of the disease within five years [34,54]. As we stated above, most colon cancer patients from our study were stage IIA (33.7%), followed by stage IIIB (22.5%). Most rectal cancer patients were stage IIA (21.2%), followed by stage I (18.7%) and stage IV (18.2%). Despite expecting a worse prognosis in rectal cancer patients, we observed that 27.8% of colon cancer and 18.0% of rectal cancer patients died from a colorectal cancer-related cause. Follow-up time ranged from 2 to 7 years and, in that period, 14.6% of the patients with colon cancer and 19.3% of the patients with rectal cancer had recurrence, mostly in the liver. This data is consistent with literature, which shows a low percentage of patients diagnosed at stage I, 13.0% for colon cancer and 18.7% for rectal cancer. Also, the percentage of stage VI diagnosed patients was very close to that observed in literature, with 18.2% for rectal cancer and 18.7% for colon cancer. From this data, we would expect a higher mortality in rectal cancer patients compared to colon cancer, but we observed very similar results, documented by using the log-rank test when doing a comparison between colon and rectum cancer survival (p=0.518). In literature, studies have shown conflicting results when comparing prognosis and localization [34]. Reduced survival in left colon cancer compared to right colon was reported in a Norwegian study from 1987 and Aldrige et al. reported similar results [34-36], but no differences were detected in other studies [34,37-39]. We also observed a lower value of 5 years disease recurrence-14.6% and 19.3% for colon and rectal cancer respectively-when compared with values of 40% found in literature. This data may reveal a different biological behaviour or be the result of the follow-up time; however, other studies with larger series must be done.
Conclusions
In the presented study, we analysed the CRC epidemiological characteristics of patients treated at Braga Hospital, Northern Portugal, in the period from January 1st 2005 to January 1st 2010. Although Portuguese epidemiological data are scarce, when comparing ours results with Brazilian and world literature data, similar results were observed. our study found that CRC was more often diagnosed in elderly and male patients and that few patients had a previous CRC personal or family history. Most patients were symptomatic at presentation and most cancers were localized in the colon, of which most was left-sided. Histological staging revealed that most of the colon and rectal cancers were stage IIA. Despite these results, we also observed that colon cancer patients had slightly higher mortality than rectal cancer patients (27.8% versus 18.0%), but a higher percentage of rectal cancer patients had recurrence during this period. This study on CRC is the first conducted in Braga, North of Portugal. This data documents the need to establish a nationwide CRC screening program, creating a better resource management and improving early diagnosis.
References
- Svagzdys S, Lesauskaite V, Pavalkis D, Nedzelskiene I, Pranys D, et al. (2009) Microvessel density as new prognostic marker after radiotherapy in rectal cancer. BMC Cancer 9: 95.
- Des Guetz G, Uzzan B, Nicolas P, Cucherat M, Morere JF, et al. (2006) Microvessel density and VEGF expression are prognostic factors in colorectal cancer. Meta-analysis of the literature. Br J Cancer 94: 1823-1832.
- Brenner H, Hoffmeister M, Haug U (2008) Should colorectal cancer screening start at the same age in European countries? Contributions from descriptive epidemiology. Br J Cancer 99: 532-535.
- Parkin DM, Bray F, Ferlay J, Pisani P (2001) Estimating the world cancer burden: Globocan 2000. Int J Cancer 94: 153-156.
- Aljebreen AM (2007) Clinico-pathological patterns of colorectal cancer in Saudi Arabia: younger with an advanced stage presentation. Saudi J Gastroenterol 13: 84-87.
- Center MM, Jemal A, Smith RA, Ward E (2009) Worldwide variations in colorectal cancer. CA Cancer J Clin 59: 366-378.
- Alves Pereira (1999) Cirurgia, Patologia e Clínica 47: 678-700.
- Henry KA, Niu X, Boscoe FP (2009) Geographic disparities in colorectal cancer survival. Int J Health Geogr 8: 48.
- Barozzi C, Ravaioli M, D'Errico A, Grazi GL, Poggioli G, et al. (2002) Relevance of biologic markers in colorectal carcinoma: a comparative study of a broad panel. Cancer 94: 647-657.
- Neagoe A, Molnar AM, Acalovschi M, Seicean A, Serban A (2004) Risk factors for colorectal cancer: an epidemiologic descriptive study of a series of 333 patients. Rom J Gastroenterol 13: 187-193.
- Zavoral M, Suchanek S, Zavada F, Dusek L, Muzik J, et al. (2009) Colorectal cancer screening in Europe. World J Gastroenterol 15: 5907-5915.
- Alexander DD, Waterbor J, Hughes T, Funkhouser E, Grizzle W, et al. (2007) African-American and Caucasian disparities in colorectal cancer mortality and survival by data source: an epidemiologic review. Cancer Biomark 3: 301-313.
- Bosetti C, Levi F, Rosato V, Bertuccio P, Lucchini F, et al. (2010) Recent trends in colorectal cancer mortality in Europe. Int J Cancer 129: 180-191.
- Carneiro Chaves F (2005) Rastreio e Prevenção dos tumores malignos do aparelho digestive.
- Instituto Português de Oncologia do Porto (2005) RORENO – Registo Oncológico Regional do Norte.
- Instituto Nacional de Estatísticas (2006) Estatísticas da Saúde 2005.
- Sunil Dolwani, Julian R. Sampson: Familial Colorectal Cancer: cap 3: 37- 58.
- Brenner H, Hoffmeister M, Arndt V, Haug U (2007) Gender differences in colorectal cancer: implications for age at initiation of screening. Br J Cancer 96: 828-831.
- Adachi CT (2009) Evolução do Carcinoma Colorectal, comparando doentes com idades acima e abaixo dos 40 anos, quanto à diferenciação tumoral e ao estadio do tumor. Rev bras Coloproct 29: 351-357.
- Habr-Gama A (2005) [Colorectal cancer: the importance of its prevention]. Arq Gastroenterol 42: 2-3.
- Boardman LA, Morlan BW, Rabe KG, Petersen GM, Lindor NM, et al. (2007) Colorectal cancer risks in relatives of young-onset cases: is risk the same across all first-degree relatives? Clin Gastroenterol Hepatol 5: 1195-1198.
- Berg M, Agesen TH, Thiis-Evensen E; INFAC-study group, Merok MA, Teixeira MR, et al. (2010) Distinct high resolution genome profiles of early onset and late onset colorectal cancer integrated with gene expression data identify candidate susceptibility loci. Mol Cancer 9: 100.
- Zafar SY, Abernethy AP, Abbott DH, Grambow SC, Marcello JE, et al. (2008) Comorbidity, age, race and stage at diagnosis in colorectal cancer: a retrospective, parallel analysis of two health systems. BMC Cancer 8: 345.
- Rougier P, Mitry E (2003) Epidemiology, treatment and chemoprevention in colorectal cancer. Ann Oncol 14 Suppl 2: ii3-5.
- Jellema P, van der Windt DA, Bruinvels DJ, Mallen CD, van Weyenberg SJ, et al. (2010) Value of symptoms and additional diagnostic tests for colorectal cancer in primary care: systematic review and meta-analysis. BMJ 340: c1269.
- Fairley TL, Cardinez CJ, Martin J, Alley L, Friedman C, et al. (2006) Colorectal cancer in U.S. adults younger than 50 years of age, 1998-2001. Cancer 107: 1153-1161.
- Imperiale TF, Kahi CJ, Stuart JS, Qi R, Born LJ, et al. (2008) Risk factors for advanced sporadic colorectal neoplasia in persons younger than age 50. Cancer Detect Prev 32: 33-38.
- Abdullah M, Sudoyo AW, Pranowo BS, Rini D, Sutrisna B, et al. (2009) Expression of NF-kappaB and COX-2 in young versus older patients with sporadic colorectal cancer. Acta Med Indones 41: 70-74.
- Xu AG, Yu ZJ, Jiang B, Wang XY, Zhong XH, et al. (2010) Colorectal cancer in Guangdong Province of China: a demographic and anatomic survey. World J Gastroenterol 16: 960-965.
- Brenner H, Altenhofen L, Hoffmeister M (2010) Sex, age, and birth cohort effects in colorectal neoplasms: a cohort analysis. Ann Intern Med 152: 697-703.
- Murphy G, Devesa SS, Cross AJ, Inskip PD, McGlynn KA, et al. (2011) Sex disparities in colorectal cancer incidence by anatomic subsite, race and age. Int J Cancer 128: 1668-1675.
- Liu LU, Holt PR, Krivosheyev V, Moss SF (1999) Human right and left colon differ in epithelial cell apoptosis and in expression of Bak, a pro-apoptotic Bcl-2 homologue. Gut 45: 45-50.
- Michelle Fraga Eisenhardt (2012) Clinical and epidemiological evaluation of patients with colorectal cancer from Rio Grande do Sul. J Coloproctol 32.
- Sjo OH, Lunde OC, Nygaard K, Sandvik L, Nesbakken A (2008) Tumour location is a prognostic factor for survival in colonic cancer patients. Colorectal Dis 10: 33-40.
- Halvorsen TB, Seim E (1987) Tumour site: a prognostic factor in colorectal cancer? A multivariate analysis. Scand J Gastroenterol 22: 124-128.
- Aldridge MC, Phillips RK, Hittinger R, Fry JS, Fielding LP (1986) Influence of tumour site on presentation, management and subsequent outcome in large bowel cancer. Br J Surg 73: 663-670.
- Jagoditsch M, Lisborg PH, Jatzko GR, Wette V, Kropfitsch G, et al. (2000) Long-term prognosis for colon cancer related to consistent radical surgery: multivariate analysis of clinical, surgical, and pathologic variables. World J Surg 24: 1264-1270.
- Angell-Andersen E, Tretli S, Coleman MP, Langmark F, Grotmol T (2004) Colorectal cancer survival trends in Norway 1958-1997. Eur J Cancer 40: 734-742.
- Wiggers T, Arends JW, Volovics A (1988) Regression analysis of prognostic factors in colorectal cancer after curative resections. Dis Colon Rectum 31: 33-41.
- Benson AB 3rd (2007) Epidemiology, disease progression, and economic burden of colorectal cancer. J Manag Care Pharm 13: S5-18.
- Ho YH, Siu SK, Buttner P, Stevenson A, Lumley J, et al. (2010) The effect of obstruction and perforation on colorectal cancer disease-free survival. World J Surg 34: 1091-1101.
- Costa RPS, Lupinacci RA (2008) Resultados do Tratamento do Câncer Colorretal (T4) Perfurado: Análise de 14 Pacientes Operados. Revista brasileira Coloproctologia 28.
- Puppa G, Sonzogni A, Colombari R, Pelosi G (2010) TNM staging system of colorectal carcinoma: a critical appraisal of challenging issues. Arch Pathol Lab Med 134: 837-852.
- Quah HM, Chou JF, Gonen M, Shia J, Schrag D, et al. (2008) Identification of patients with high-risk stage II colon cancer for adjuvant therapy. Dis Colon Rectum 51: 503-507.
- Merkel S, Wein A, Günther K, Papadopoulos T, Hohenberger W, et al. (2001) High-risk groups of patients with Stage II colon carcinoma. Cancer 92: 1435-1443.
- Derwinger K, Kodeda K, Bexe-Lindskog E, Taflin H (2010) Tumour differentiation grade is associated with TNM staging and the risk of node metastasis in colorectal cancer. Acta Oncol 49: 57-62.
- Saad-Hossne R (2005) Estudo retrospective de pacientes portadores de cancro colorectal atendidos na faculdade de medicina de Botucatu no período 2000-2003. Rev bras Coloproct 25: 31-37.
- Carneiro Neto JD (2006) Cancer Colorectal: características clinicas e anatomopatológicas em pacientes com idade inferior a 40 anos. Rev bras Coloproct 26: 430-435.
- Washington MK (2008) Colorectal carcinoma: selected issues in pathologic examination and staging and determination of prognostic factors. Arch Pathol Lab Med 132: 1600-1607.
- Sternberg A, Amar M, Alfici R, Groisman G (2002) Conclusions from a study of venous invasion in stage IV colorectal adenocarcinoma. J Clin Pathol 55: 17-21.
- Kingston EF, Goulding H, Bateman AC (2007) Vascular invasion is underrecognized in colorectal cancer using conventional hematoxylin and eosin staining. Dis Colon Rectum 50: 1867-1872.
- Labianca R, Beretta GD, Mosconi S, Pessi MA, Milesi L (2005) The development of clinical research in CRC. Ann Oncol 16 Suppl 4: iv37-43.
- Gurzu S, Jung J, Azamfirei L, Mezei T, Cîmpean AM, et al. (2008) The angiogenesis in colorectal carcinomas with and without lymph node metastases. Rom J Morphol Embryol 49: 149-152.
- Calvo HJ, Ortega GD, Pardo RJM, López MAJ, Cubo T (2000) Biologia molecular del processo metastásico del cancer colorectal. Cirugia Española 68: 577-587.
- Sun LC, Chu KS, Cheng SC, Lu CY, Kuo CH, et al. (2009) Preoperative serum carcinoembryonic antigen, albumin and age are supplementary to UICC staging systems in predicting survival for colorectal cancer patients undergoing surgical treatment. BMC Cancer 9: 288.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 14977
- [From(publication date):
November-2013 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 10382
- PDF downloads : 4595