Research Article Open Access
A Highly Sensitive LC-Ms/Ms Method for Determination of Desvenlafaxine in Rabbit Plasma and its Application to Rabbit Pharmacokinetic Study
Shashidhar Reddy D1*, Prakash Rao2 and Devaraj VC11Department of Pharmacology, Bioneeds Preclinical Services, Bangalore, India
2Department of Pharmaceutics, Karnataka College of Pharmacy, Bangalore, India
- *Corresponding Author:
- Dr. Shashidhar Reddy.D
Research Scholar
Dept. of Pharmacology
Bioneeds Laboratory Animals & Preclinical Services
Bangalore: 562111, Karnataka, India
Tel: +91 8374093233, +91 8453336609
Fax: 914067204199
E-mail: dasdev@in.com, devcology@yahoo.com
Received date: September 07, 2011; Accepted date: October 07, 2011; Published date: October 25, 2011
Citation: Reddy SD, Rao P, Devaraj VC (2011) A Highly Sensitive LC-Ms/Ms Method for Determination of Desvenlafaxine in Rabbit Plasma and its Application to Rabbit Pharmacokinetic Study. J Anal Bioanal Tech 2:125. doi: 10.4172/2155-9872.1000125
Copyright: © 2011 Reddy SD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
A highly senstive and rapid LC-MS/MS method has been developed and validated for the estimation of desvenlafaxine in rabbit plasma. The chromatographic separation was performed with 0.2% formic acid: methanol at flow rate of 0.4 mL/min on Symmetry Shield RP18 column with a total run time of 3.0 min. TheMS/MS ion transitions monitored were 263.90? 58.10 for desvenlafaxine and 281.30--> 86.10 for IS (metaprolol). Method validation and pre-clinical sample analysis were performed as per FDA guide lines and the results met the acceptance crieteria. The lower limit of quantification achieved was 0.5 ng/mL and the linearity was observed from 0.5 to 1500 ng/mL. This novel method has been applied to pharmacokinetic study of desvenlafaxine in rabbits.
Keywords
LC-MS/MS; Desvenlafaxine; Pharmacokinetics; Rabbit plasma
Introduction
Desvenlafaxine (DVS) is an novel serotonin (5-HT) and norepinephrine (NE) reuptake inhibitor (SNRI) that is currently in clinical development for the treatment of major depressive disorder and vasomotor symptoms associated with menopause. Desvenlafaxine (O-desmethyl venlafaxine, Figure 1) is the major active metabolite of the antidepressant venlafaxine, a medication used to treat major depressive, generalized anxiety and panic disorders [1]. Literature survey revealed that few LC-MS methods have been reported for estimation of DVS in biological matrices. Although the methods are sensitive and has an efficient extraction procedure but the total chromatographic run time is too long (10 min), whichmay not be favourable for routine subject analysis. Also, all these reported procedures have a very high oncolumn loading of the analytes at the ULOQ level, which may reduce the efficiency of the column and may affect the column life [2-4].
Methods capable of separating enantiomers of venlafaxine and O-desmethylvenlafaxine have been reported based on capillary electrophoresis [5-13] and HPLC-ESI/MS [14]. Thus, the aim of the present work was to establish a simple, accurate, rapid and sensitive LC-MS/MS method with a lower limit of quantification (LLOQ) of 0.5 ng/mL using a very low volume (50 µL) of rabbit plasma. Our method involves simple liquid-liquid extraction sample processing using terbutyl methyl ether (amicable for plasma) and has a run time of 3.0 min for the separation of both the analyte and IS; hence our method gives higher throughput. The newly developed LC-MS/MS method was successfully used in a rabbit pharmacokinetic study and to assess the plasma concentration of DVS following administration of a 50 mg/kg oral dose.
Experimental
Chemicals and reagents
Desvenlafaxine succinate (DVS) and metaprolol (IS) were procured from Dr Reddy laboratory Pvt Ltd (Hyderabad, India). HPLC-grade acetonitrile and methanol were purchased from Rankem (Ranbaxy Fine Chemicals Limited, New Delhi, India). Analytical grade formic acid was purchased from S.D. Fine Chemicals (Mumbai, India). Albino male rabbits were purchased from Reliance Life Sciences (Mumbai, India).
Hplc operating conditions
A Shimadzu VP (Shimadzu, Japan) LC system equipped with degasser (G1379A), quaternary pump (10ADvp), column oven (CTO- 10ASvp) and auto-sampler (SIL-HTC) along with a system controller (SCL-10Avp) was used to inject 2 µL aliquots of the processed samples on a Symmetry Shield RP18 column (50 x 4.6 mm, 3.5 µm, Waters Corporation, Ireland, UK), which was kept at ambient temperature (24 ± 2°C). The isocratic mobile phase, a mixture of 0.2% formic acid and methanol mixture (20:80, v/v) was filtered through a 0.45 µm membrane filter (Millipore) and then degassed ultrasonically for 5 min was delivered at a flow rate of 0.40 mL/min into the mass spectrometer electro spray ionization chamber.
Mass spectrometry operating conditions
Quantification was achieved by MS/MS detection in positive ion mode for analyte and IS using a MDS Sciex (Foster City, CA, USA) API 4000 mass spectrometer, equipped with a TurboionsprayT interface at 500°C. The common parameters, i.e. curtain gas, nebulizer gas, auxillary gas and collision gas, were set at 10, 35, 40 and 6 psi, respectively. The compounds parameters, i.e. declustering potential (DP), collision energy (CE), collision exit potential (CXP) and entrance potential (EP) for DVS and IS were 60, 43, 8, 10 V and 44, 26, 8, 10 V, respectively. Detection of the ions was performed in the multiple reaction monitoring (MRM) mode, monitoring the transition of the m/z 263.90 precursor ion to the m/z 58.10 product ion for DVS and m/z 281.30 precursor ion to the m/z 86.10 product ion for IS. Quadrupole Q1 was set on low resolution where as Q3 was set on unit resolution. The analytical data were processed by Analyst software (version 1.4.2).
Preparation of stock and standard solutions
Primary stock solutions of DVS for preparation of standard and quality control (QC) samples were prepared from separate weighing. The primary stock solutions were prepared in methanol (1000 µg/mL). The IS stock solution of 1000 µg/mL was prepared in methanol. The stock solutions of DVS and IS were stored at 4°C, which were found to be stable for one month (data not shown) and successively diluted with methanol to prepare working solutions to prepare the calibration curve (CC). Another set of working stock solutions of DVS were made in methanol (from primary stock) for preparation of QC samples. Working stock solutions were stored at approximately 4°C for a week (data not shown). Appropriate dilutions of DVS stock solution were made in methanol to produce working stock solutions of 0.50, 1.00, 10, 50, 100, 500, 750 and 1500 ng/mL. Working stocks were used to prepare plasma calibration standards. A working IS solution (200 ng/ mL) was prepared in methanol. Calibration samples were prepared by spiking 45 µL of control rabbit plasma with the appropriate working solution of the analyte (5 µL) and IS (10 µL) on the day of analysis. Samples for the determination of precision and accuracy were prepared by spiking control rabbit plasma in bulk with DVS at appropriate concentrations (0.50, 1.50, 500 and 800 ng/mL) and 50 µL plasma aliquots were distributed into different tubes. All the samples were stored at -80 ± 10°C.
Recovery
The efficiency of DVS and IS extraction from rabbit plasma was determined by comparing the responses of the analytes extracted from replicate QC samples (n = 6) with the response of analytes from post extracted plasma standard sample at equivalent concentrations by liquid-liquid extraction [15]. Recoveries of DVS were determined at QC low and QC high concentrations, i.e. 1.50 and 800 ng/mL, whereas the recovery of the IS was determined at a single concentration of 200 ng/mL.
Sample preparation
A simple liquid-liquid extraction method was followed for extraction of DVS from rabbit plasma. To an aliquot of 50 µL plasma, IS solution (10 µL of 200 ng/mL) was added and mixed for 15 s on a cyclomixer (Remi Instruments, Mumbai, India). After the addition of 2 mL of ter-butyl methyl ether (TBME), the mixture was vortexed for 2 min, followed by centrifugation for 10 min at 3200 rpm on Multifuge 3SR (Heraus, Germany). The organic layer (1.8 mL) was separated and evaporated to dryness at 40°C using a gentle stream of nitrogen (Turbovap®, Zymark® Kopkinton, MA, USA). The residue was reconstituted in 200 µL of the mobile phase and 2 µL was injected onto LC-MS/MS system.
Validation procedures
A full validation according to the FDA guidelines (US DHHS, FDA, CDER, 2001) was performed for the assay in rabbit plasma [16].
Specificity and selectivity: The specificity of the method was evaluated by analyzing rabbit plasma samples from at least six different lots to investigate the potential interferences at the LC peak region for analyte and IS.
Matrix effect: The effect of rabbit plasma constituents over the ionization of DVS and IS was determined by comparing the responses of the post extracted plasma QC samples (n = 6) with the response of analytes from neat standard samples (5 µL of required working stock sample spiked into 45 µL of methanol instead of blank plasma) at equivalent concentrations [17]. Matrix effect was determined at low and high concentrations, i.e. 1.50 and 800 ng/mL, whereas the matrix effect over the IS was determined at a single concentration of 200 ng/ mL.
Calibration curve: The eight point calibration curve (0.50, 1.00, 10, 50, 100, 500, 750 and 1500 ng/mL) was constructed by plotting the peak area ratio of DVS: IS against the nominal concentration of calibration standards in rabbit plasma. Following the evaluation of different weighing factors, the results were fitted to linear regression analysis with the use of 1/X2 (X = concentration) weighting factor. The calibration curve had to have a correlation coefficient (r) of 0.99 or better. The acceptance criteria for each back-calculated standard concentration were ± 15% deviation from the nominal value except at LLOQ, which was set at ± 20% (US DHHS, FDA, CDER, 2001).
Precision and accuracy: The intra-assay precision and accuracy were estimated by analyzing six replicates containing DVS at four different QC levels: 0.50, 1.50, 500 and 800ng/mL in plasma. The interassay precision was determined by analyzing the four levels QC samples on four different runs. The criteria for acceptability of the data included accuracy within ± 15% deviation (SD) from the nominal values and a precision of within ± 15% relative standard deviation (RSD) except for LLOQ, where it should not exceed ± 20% (US DHHS, FDA, CDER, 2001).
Stability experiments: The stability of DVS and IS in the injection solvent was determined periodically by injecting replicate preparations of processed plasma samples for up to 12 h (in the autosampler at 4°C) after the initial injection. The peak-areas of the analyte and IS obtained in the initial cycle were used as the reference to determine the stability at subsequent points. Stability of DVS in plasma during 6 h (bench-top) was determined at ambient temperature (24 ± 2°C) at two concentrations (1.50 and 800 ng/mL) in six replicates. Freezer stability of DVS in rabbit plasma was assessed by analyzing the LQC and HQC samples stored at -80 ± 10°C for at least 30 days. The stability of DVS in rat plasma following three freeze-thaw cycles was assessed using QC samples spiked with DVS. The samples were stored at -80 ± 10°C between freeze-thaw cycles. The samples were thawed by allowing them to stand (unassisted) at room temperature for approximately 2 h. The samples were then returned to the freezer. The samples were processed using the same procedure as described in the Sample Preparation section. Samples were considered stable if assay values were within the acceptable limits of accuracy (i.e. ±15% SD) and precision (i.e. ±15% RSD).
In vivo studies in rabbits
A pharmacokinetic (PK) study was performed in over night (~12 h) fasted healthy male albino rabbits (n = 3, weight range 2.3-2.5 kg) following approval from the ethical committee. During fasting time animals had free access to water. Blood samples were obtained following oral administration of 50 mg/kg DVS, (in the form of a suspension and extended release tablets with 50 mg equivalent weight of DVS, via silicone rubber gastric intubation tube to their respective groups) into polypropylene tubes containing EDTA solution as an anti-coagulant at pre-dose, 0.25, 0.5, 1, 2, 4, 6, 8 and 24 h. Plasma was harvested by centrifuging the blood using Biofuge (Hereaus, Germany) at 1760 g for 5 min and stored frozen at -80 ± 10°C until analysis. Plasma (50 µL) samples were spiked with IS and processed as described above. Along with PK samples, QC samples at low, medium and high concentration were assayed in duplicate and were distributed among calibrators and unknown samples in the analytical run; not more than 33% of the QC samples were greater than ± 15% of the nominal concentration. Plasma concentration-time data of DVS was analyzed by non-compartmental method using WinNonlin Version 5.1 (Pharsight Corporation, Mountain View, CA, USA).
Results and Discussion
Liquid chromatography
The feasibility of various mixture(s) of solvents such as acetonitrile and methanol using different buffers such as ammonium acetate, ammonium formate and formic acid along with altered flow rates (in the range of 0.1-0.5 mL/min) was tested for complete chromatographic resolution of DVS and IS (data not shown). The resolution of peaks was achieved with 0.2% formic acid: methanol (20:80, v/v) with a flow rate of 0.40 mL/min, on a Symmetry Shield RP18 column (50 × 4.6 mm, 3.5 µm, Waters, UK) and was found to be suitable for the determination of electrospray response for DVS and IS.
Mass spectroscopy
In order to optimize ESI conditions for DVS and IS, quadrupole full scans were carried out in positive ion detection mode. During a direct infusion experiment, the mass spectra for DVS and IS revealed peaks at m/z 263.90 and 281.30, respectively, as protonated molecular ions, [M + H]. Following detailed optimization of mass spectrometry conditions (provided in the Instrumentation and Chromatographic Conditions section) the m/z 263.90 precursor ion to the m/z 58.10 was used for quantification of DVS. Similarly, for IS the m/z 281.30 precursor ion to the m/z 86.10 was used for quantification purpose.
Recovery
A simple liquid-liquid extraction with TBME proved to be robust and provided the cleanest samples. The results of the comparison of neat standards vs plasma-extracted standards were estimated for DVS at 1.50 and 800 ng/mL and the mean recovery was found to be 70.31 ± 5.58 and 67.37 ± 2.44%, respectively. The recovery of IS at 200 ng/mL was 88.32 ± 6.92%.
Matrix effect, specificity and selectivity
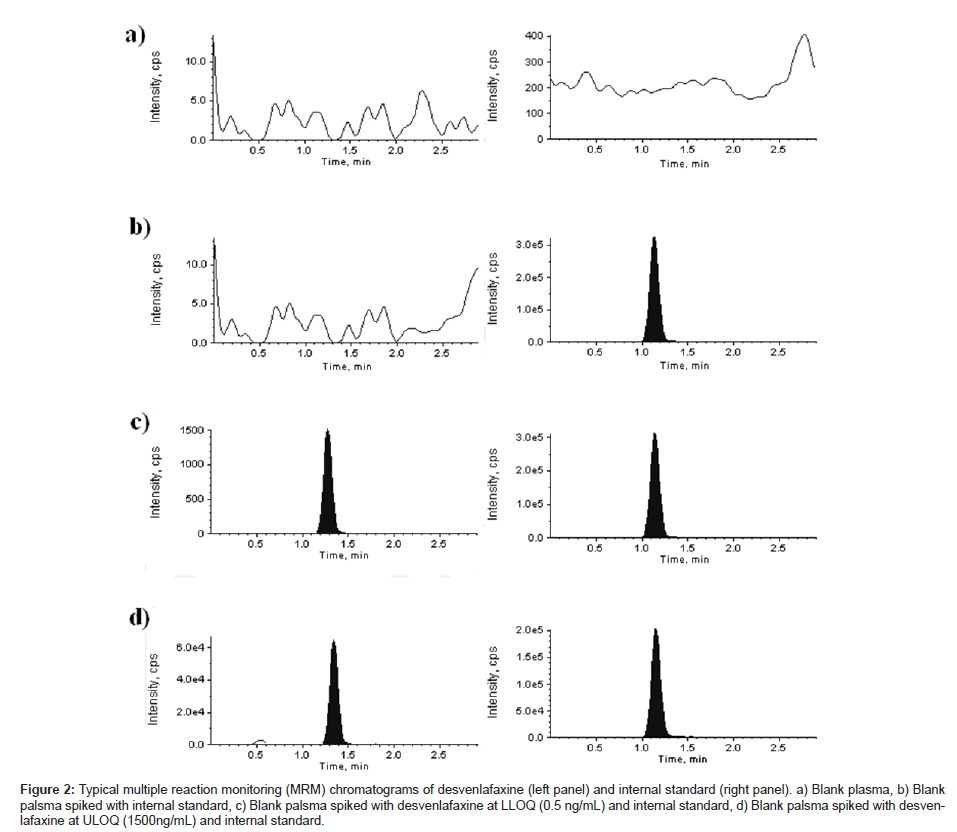
Average matrix factor values (matrix factor = response of post spiked concentrations/response of neat concentrations) obtained were +0.60 (CV: 3.21%, n = 6) and +0.71 (CV: 12.70%, n = 6) for DVS in rabbit plasma at QC low (1.50 ng/mL) and QC high (800 ng/ mL) concentrations, respectively. No signifi cant peak area differences were observed. The matrix effect on IS was found to be +1.16 (CV: 14.95%, n = 6) at the tested concentration of 200 ng/ mL. Overall it was found that the plasma extract has a small impact on the ionization of analyte and IS. Figure 2 shows a typical chromatogram for the control rabbit plasma (free of analyte and IS), control rabbit plasma spiked with IS, rabbit plasma spiked with DVS at lower limit of quantification (LLOQ) and IS and rabbit plasma spiked with DVS at upper limit of quantification (ULOQ) and IS. No interfering peaks from endogenous compounds are observed at the retention times of analyte and IS in the matrix. The retention times of DVS and IS were 1.43 and 1.12 min, respectively. The total chromatographic run time was 3.0 min.
Figure 2: Typical multiple reaction monitoring (MRM) chromatograms of desvenlafaxine (left panel) and internal standard (right panel). a) Blank plasma, b) Blank palsma spiked with internal standard, c) Blank palsma spiked with desvenlafaxine at LLOQ (0.5 ng/mL) and internal standard, d) Blank palsma spiked with desvenlafaxine at ULOQ (1500ng/mL) and internal standard.
Calibration curve
The plasma calibration curve was constructed using eight calibration standards (0.5-1500 ng/mL). The calibration standard curve had a reliable reproducibility over the standard concentrations across the calibration range. The calibration curve was prepared by determining the best fit of peak-area ratios (peak area analyte/peak area IS) vs concentration, and fitted to the y = mx + c using a weighing factor (1/X2). The average regression (n = 4) was found to be =0.998. The lowest concentration with the RSD < 20% was taken as LLOQ and was found to be 0.5ng/mL. The percentage accuracy observed for the mean of back-calculated concentrations for four calibration curves for DVS was within 88.4-110, while the precision (%CV) values ranged from 0.47 to 9.08.
Accuracy and precision
Accuracy and precision data for intra- and inter-day plasma samples are presented in (Table 1). The assay values on both the occasions (intra- and inter-day) were found to be within the accepted variable limits.
| Intra-day variation (six replicates at each concentration) | |||||
|---|---|---|---|---|---|
| Theoretical concentration (ng/mL) | Run | Measured concentration (ng/mL) | |||
| Mean | SD | RSD | Accuracy % | ||
| 0.50 | 1 | 0.53 | 0.01 | 1.89 | 116 |
| 2 | 0.59 | 0.08 | 13.6 | 105 | |
| 3 | 0.49 | 0.09 | 18.4 | 98.2 | |
| 4 | 0.55 | 0.05 | 9.09 | 103 | |
| 1.50 | 1 | 1.48 | 0.03 | 2.03 | 96.5 |
| 2 | 1.57 | 0.07 | 4.46 | 104 | |
| 3 | 1.53 | 0.06 | 3.92 | 102 | |
| 4 | 1.61 | 0.02 | 1.24 | 112 | |
| 500 | 1 | 498 | 2.51 | 0.50 | 98.5 |
| 2 | 508 | 2.14 | 0.42 | 107 | |
| 3 | 512 | 5.41 | 1.06 | 101 | |
| 4 | 507 | 6.97 | 1.37 | 92.6 | |
| 800 | 1 | 821 | 5.90 | 0.72 | 107 |
| 2 | 805 | 3.85 | 0.48 | 97.6 | |
| 3 | 816 | 8.21 | 1.01 | 105 | |
| 4 | 829 | 7.31 | 0.88 | 113 | |
| Inter-day variation (twenty four replicates at each concentration) | |||||
| Theoretical concentration (ng/mL) | Mean | SD | RSD | Accuracy % | |
| 0.50 | 0.53 | 0.09 | 17.0 | 104 | |
| 1.50 | 1.51 | 0.08 | 5.30 | 98.7 | |
| 500 | 512 | 6.71 | 1.31 | 101 | |
| 800 | 823 | 9.87 | 1.20 | 109 | |
RSD, Relative standard deviation (SD × 100/mean).
Table 1: Intra- and inter-day precision of determination of DVS in rabbit plasma.
Stability
The predicted concentrations for DVS at 1.50 and 800 ng/mL samples deviated within ±15% of the nominal concentrations in a battery of stability tests: in-injector (12 h), bench-top (6 h), repeated three freeze-thaw cycles and freezer stability at -80 ± 10°C for at least for 30 days (Table 2). The results were found to be within the assay variability limits during the entire process.
| Nominal concentration (ng/mL) | Stability | Mean ± SD, n = 6 (ng/mL) | Accuracy (%) | Precision (%CV) |
|---|---|---|---|---|
| 1.50 | 0 h | 1.51 ± 0.03 | 98.2 | 3.52 |
| Third freeze–thaw | 1.60 ± 0.05 | 108 | 3.38 | |
| 6 h (bench-top) | 1.45 ± 0.06 | 93.5 | 4.15 | |
| 12 h (in-injector) | 1.41 ± 0.04 | 90.2 | 2.57 | |
| 30 days at −80°C | 1.55 ± 0.02 | 95.2 | 3.87 | |
| 800 | 0 h | 804± 5.54 | 114 | 8.74 |
| Third freeze–thaw | 795± 10.5 | 97.5 | 4.58 | |
| 6 h (bench-top) | 813± 7.81 | 103 | 2.34 | |
| 12 h (in-injector) | 805± 5.78 | 98.7 | 4.08 | |
| 30 days at −80°C | 785± 7.87 | 101 | 4.62 |
Table 2: Stability data of DVS quality controls in rabbit plasma.
In vivo Studies
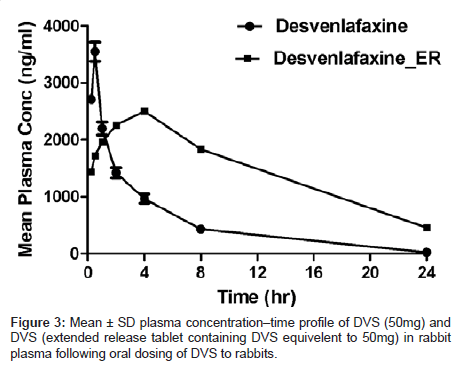
The sensitivity and specificity of the assay were found to be sufficient for accurately characterizing the plasma pharmacokinetics of DVS in rabbits. Profiles of the mean plasma concentration vs time were shown in (Figure 3). Pharmacokinetic parameters were tabulated in (Table 3). Maximum concentration in plasma (Cmax 3550 ± 4.31 ng/ mL, 2500 ± 6.15 ng/mL) was achieved at (0.5 h, 4.0h) Tmax respectively for DVS suspension and DVS extended release tablets. The AUC(0-t) was (13190 ± 18.1h*ng /mL, 35397 ± 12.6h*ng /mL) respectively for DVS suspension and DVS extended release tablets. The higher sensitivity of this method compared with the current existing methods in literature facilitates the quantification of DVS at lower concentrations with high turnover.
| PK Parameters | Desvenlafaxine | Desvenlafaxine _ER |
|---|---|---|
| Cmax (ng/mL) | 3550±4.31 | 2500±6.15 |
| Tmax (hr) | 0.51 | 4.00 |
| AUClast (hr*ng/mL) | 13190±18.1 | 35397±12.6 |
Table 3: Mean ± SD pharmacokinetic parameters of DVS (50mg) and DVS (extended release tablet containing DVS equivalent to 50mg) following oral dosing of DVS in rabbits.
Conclusion
A method using LC-MS/MS for the determination of DVS in rabbit plasma employing simple liquid-liquid extraction was developed. The method is rapid, simple, specific and sensitive, and additionally demonstrates good accuracy and precision. Compared with the published methods, the present method features high selectivity and sensitivity with an LLOQ of 0.5 ng/ mL. We believe that this highthroughput method could provide a useful tool for the determination of DVS in plasma. The established method was successfully applied to a rabbit pharmacokinetic study and to assess the plasma concentration.
Acknowledgements
The authors are thankful to Dr. Vinay babu, (MD), Bioneed preclinical services Pvt Ltd. for providing facilities to carry out the work and for his valuable suggestions during the work.
References
- Richard PP, Manouchkathe CP (2009) Desvenlafaxine: A new serotonin-norepinephrine reuptake inhibitor for the treatment of adults with major depressive disorder. Clin Therap 31: 1374-1404.
- Castro AD, Concheiro M, Quintela O, Cruz A, Lopez AM (2008) LC-MS/MS method for the determination of nine antidepressants and some of their main metabolites in oral fludi and plasma. Study of correlation between venlafaxine concentrations in both matrices. J Pharm Bio Anal 48: 183-193.
- Desiderio Z, Aturki S, Fanali S (2001) Use of vancomycin silica stationary phase in packed capillary electrochromatography I. Enantiomer separation of basic compounds. Electrophoresis 22: 535-543.
- Cherkaoui S, Rudaz S, Varesio E, Veuthey JL (2001) On-line capillary electrophoresis- electrospray mass spectrometry for the stereoselective analysis of drugs and metabolites. Electrophoresis 22: 3308-3315.
- Rudaz S, Stella C, Balant-Gorgia AE, Fanali S, Veuthey JL (2000) Simultaneous stereoselective analysis of venlafaxine and O-desmethylvenlafaxine enantiomers in clinical samples by capillary electrophoresis using charged cyclodextrins. J Pharm Biomed Anal 23: 107-115.
- Fanali S, Rudaz S, Veuthey JL, Desiderio C (2001) Use of vancomycin silica stationary phase in packed capillary electrochromatography II. Enantiomer separation of venlafaxine and O-desmethylvenlafaxine in human plasma. J Chromatogr A 919: 195-203.
- Castaing N, Titier K, Receveur DM, Le-Deodic M, Le-bars D, et al. (2007) Quantification of eight new antidepressants and five of their active metabolites in whole blood by high-performance liquid chromatography-tandem mass spectrometry. J Anal Toxicol 31: 334-341.
- Bhavin NP, Naveen S, Mallika S, Pranav SS (2008) Liquid chromatography tandem mass spectrometry assay for the simultaneous determination of venlafaxine and O-desmethylvenlafaxine in human plasma and its application to a bioequivalence study. J Pharm Biomed Anal 47: 603-611.
- Wille SMR, Maudens KE, Peteghem CHV, Lambert WEE (2005) Development of a solid phase extraction for 13 'new' generation antidepressants and their active metabolites for gas chromatographic- mass spectrometric analysis. J Chromatogr A 1098: 19-29.
- Gunnar T, Mykkanen S, Ariniemi K, Lillsunde P (2004) Validated semiquantitative/ quantitative screening of 51 drugs in whole blood as silylated derivatives by gas chromatography-selected ion monitoring mass spectrometry and gas chromatography electron capture detection. J Chromatogr B 806: 205-219.
- Long C, Crifasi J, Magnin D, Graham M, Teas S (1997) Comparison of analytical methods in the determination of two venlafaxine fatalities. J Anal Toxicol 21: 166-169.
- Sauvage FL, Gaulier JM, Lachâtre G, Marquet P (2006) A fully automated turbulent-flow liquid chromatography-tandem mass spectrometry technique for monitoring antidepressants in human serum. Therap Drug Mon 28: 123-130.
- Liu W, Wang F, Li H (2007) Simultaneous stereoselective analysis of venlafaxine and O-desmethylvenlafaxine enantiomers in human plasma by HPLC-ESI/ MS using a vancomycin chiral column. J Chromatogr B Analyt Technol Biomed Life Sci 850: 183-189.
- Filiz T, Ipek O, Tamer G (2010) In-vitro and in-vivo evaluation of oral tablet formulations prepared with ketoconazole and hydroxypropyl-ß-cyclodextrin. Drug Delivery 17: 152-157.
- Dams R, Huestis MA, Lambert WE, Murphy CM (2003) Matrix effect in bioanalysis of illicit drugs with LC-MS/MS: infl uence of ionization type, sample preparation, and biofluid. J Am Soc Mass Spectrom 14: 1290-1294.
- US DHHS, FDA, CDER. Guidance for Industry: Bioanalytical Method Validation. US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Veterinary Medicine, 2001. Available from: http://www/fda.gov/cder/guidance/index.htm
- Hubert Ph, Chiap P, Crommen J, Boulanger B, Chapuzet E, et al. (1999) The SFSTP guide on the validation of chromatographic methods for drug bioanalysis Washington conférence to the laboratory. Anal Chimi Act 391: 135-148.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15435
- [From(publication date):
December-2011 - Apr 07, 2025] - Breakdown by view type
- HTML page views : 10723
- PDF downloads : 4712