Prognostic Significance of Fhit and Wwox Proteins Expression in Laryngeal Cancer
Received: 10-Oct-2013 / Accepted Date: 11-Nov-2013 / Published Date: 19-Nov-2013 DOI: 10.4172/2161-119X.1000148
Abstract
Objective: The tumor suppressor genes, Fragile Histidine Triad (FHIT) and WW domain-containing oxidoreductase (WWOX) are encoded by fragile loci FRA3B and FRA16D at chromosomes 3p14.2 and 16q23.3. The expression of their protein products has been shown to decrease in several types of cancers. This concordant loss of expression suggests that these common fragile regions of the genome, where certain environmental and/or chemical factors result in damage, have important functions in carcinogenesis. The aim of this study was to determine the prognostic significance of the expression of the FHIT and WWOX genes in laryngeal cancer.
Materials and methods: Sixty-two patients who underwent surgery for laryngeal cancer were investigated for this study. Factors taken into consideration are: age, gender, tumor stage, presence of lymph node metastasis, presence of distance metastasis, location of the tumor, tumor infiltration, length of clinical follow-up, length of remission period, the presence or absence of postoperative supplementary treatment, histopathologic differentiation of the tumor, volume of tumor, cartilage invasion, the presence of perineural invasion, and the condition of surgical resection margins have been detected. The obtained results were compared to the immune histochemistry findings.
Results: Loss of FHIT expression was demonstrated in 39 (62.9%) patients and loss of WWOX expression was demonstrated in 29 (46.8%) patients. There were statistically significant differences between FHIT distributions of the T-stages (p:0.04). FHIT reduction was associated with tumor infiltration (p=0.05). It was also associated with lymph node metastasis (p=0.012). FHIT expression significantly decreased in patients with a history of smoking (p=0.006). There was a statistically significant difference between WWOX expression and the tumor stage (p:0.036), but WWOX expression was not associated with tumor infiltration, lymph node metastasis or smoking history. The other parameters, namely perineural invasion, surgery margins, cartilage involvement, postoperative therapy, disease-free survival, and the tumor’s location did not correlate with WWOX and FHIT expression.
Conclusion: These results showed that the FHIT and WWOX genes may play a role in the prognosis of laryngeal cancer.
Keywords: FHIT, WWOX, Larengeal cancer, Metastas
255143Introduction
After skin cancer, laryngeal cancer is the second most common cancer in the head and neck region [1]. There are various treatment methods for cancer treatment; however, patients’ responses to these treatments may not always be the same. It is still unknown why one treatment may be sufficient to treat a tumor in a specific anatomic region and in a specific phase in some patients, whereas in other patients the same treatment can result in relapse, or recurrent or distant metastasis.
In the detailed analyses of the underlying reasons for such discrepancies, the focus shifted to prognostic factors that vary with respect to the patient [2].
The differences with respect to age and gender in patients with laryngeal cancer emerge in the formation of ideas related to prognostic factors. Later, the second point of emphasis has been the consumption of tobacco and alcohol in the relationship between cancer and the patient [2].
Parallel to the technological advancements and increasing number of relevant studies, subjects such as tumor phase, tumor location, vocal cord mobility, and preoperative tracheostomy opening have garnered attention [2].
For the last ten years, the new area of focus has increasingly shifted to molecular and genetic disorders in cancer patients. To illustrate, various studies have pointed to the fact that, similar to other regional cancers, oncogenes and tumor suppressor genes are major prognostic factors in laryngeal cancer, and research on this connection has been allotted a significant space in relevant literature [3]. It has recently been discovered that a short limb (3p) of the third chromosome located in the FRA16D region is the most densely affected region, and the deletions observed there are common to many other cancer types. Hence, it has been suggested that this region is the location of suppressor genes for various tumors. The FHIT gene, which constitutes one of the major subjects of current study, is also located in this region. The WWOX gene, which is a tumor suppressor gene that can be altered by oncogenic viruses, is also located in this region.
The FHIT and WWOX suppressor genes have been explicitly detected in certain organ cancers, specifically in cancers of the breast, esophagus, lung, bladder and cervix [4-6].
In the present study, FHIT and WWOX protein expressions in the pathology preparates obtained from specimens of patients with laryngeal cancer have been examined, and the relationship between these gene expressions and the possibility of relevant clinical and pathological prognostic data has been explored. Randomly selected patients diagnosed with squamous cell carcinoma who underwent surgery in our clinic for laryngeal cancer were included in this study, TMA technique was used and in all cases the expression status of immunohistochemical markers were analyzed. We were looking forward to assessing relationship between FHIT-WWOX and larinx cancer.
Materials and Methods
Patient features
Sixty-two randomly selected patients that we have followed for ten years diagnosed with squamous cell carcinoma who underwent surgery for laryngeal cancer were included in this study. The study group included 1 female patient and 61 male patients. 52 (84%) patient’s live but 10 (16%) patients are dead (because of larynx cancer).
We took specimen in sterile condition from the main cancer specimen in the operation room.
The patients’ file data were broken down into the following factors: age, gender, tumor stage, tumor location (glottik, supraglottic, transglottic), presence of lymph node metastasis, presence of distance metastasis, tumor location, length of clinical follow-up, length of remission period, presence or absence of postoperative supplementary treatment, histopathologic differentiation of the tumor, volume of tumor, cartilage invasion, the presence of perineural invasion and the condition of surgical resection margins. The obtained results were then compared to their immunohistochemistry findings.
The remission period was measured as the period between detected occurrences. Local recurrence, regional recurrence, and distance metastasis have been aggregated as relapses. Each patient or relatives signed an informed consent to join to the study.
Histopathologic and immunohistochemical analyses
Preparation of blocks: In this study group, based on the planned immunohistochemistry of the patient group in this study, the widely used Tissue Microarray (TMA) technique that enables examining more than one patient in one stage was utilized. TMA is the process of taking tissue cores from paraffin blocks using needles with diameters of 0.6 mm, 1 mm, 1.5 mm, and 2 mm, and embedding those tissues in a single paraffin block as an array (Figure 1a,1b and 1c). By using this process, almost 1,000 tissue samples can be embedded in a single paraffin block. Thus, TMA enables the analysis of hundreds of tissue samples by molecular and immunohistochemical markers.
In all the obtained TMA sections, complete surface sections have been gathered from the paraffin blocks of the patients whose tumors decreased or disappeared in the serial sections applied over TMA block or whose sections fallen due to tissue follow-up and detection artifact or technical causes during the phase of immunohistochemical dyeing and in all cases the expression status of immunohistochemical markers were analyzed.
Immunohistochemistry:
Methodology: Immunohistochemical analyses of WWOX and FHIT expression were performed in sections that were prepared within sections of paraffin blocks of tissues fixed in a formalin solution of 10%. A minimum of two preparates was examined for each patient and the tumor regions were marked.
Following the verification of the presence of a carcinoma by preparing an H+E section from multiple tissue blocks, new tissue samples were taken in order to conduct immunohistochemical analyses.
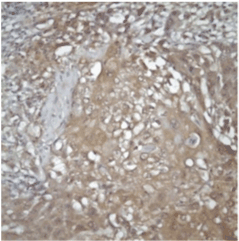
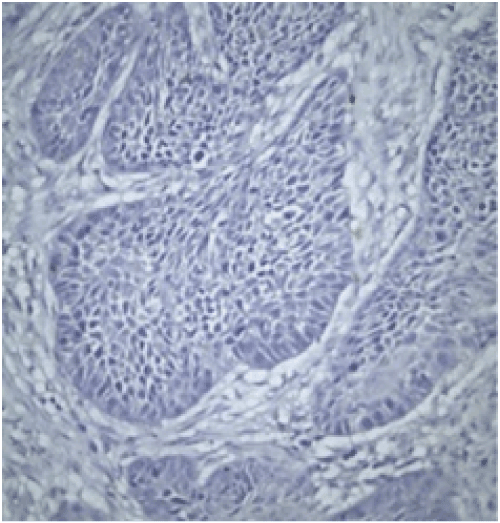
Evaluation: All evaluations have been made without referring to the clinical data of cases. Since previous studies have shown that the expression of FHIT and WWOX proteins is heterogeneous in tumors, an evaluation of FHIT and WWOX in tumor tissues was conducted by separately measuring both intensity and prevalence of dyeing. Cytoplasmic dyeing is accepted as a positive indicator. When the scores of two blind biopsies of the same case were different, the higher score was taken as the valid result. Poor cytoplasmic dyeing was graded as 1, medium cytoplasmic dyeing was graded as 2, and strong cytoplasmic dyeing was graded as 3. In evaluating dyeing prevalence, dyeing less than 10% was given 1 point, dyeing with 11-25% was given 2 points, dyeing with 26-50% was given 3 points, dyeing with 51-75% was given 4 points, dyeing with 76-100% was given 5 points. The final score was calculated after multiplying these two scores. Cases in which the multiplication value was 6 or above was classified as cases with minimal expression loss. Values lower than 6 were classified as cases with visible expression loss (Figure 2 and 3).
Statistical evaluation
The computer program SPSS 15.0 was used for statistical evaluation. Research data were also analyzed with the chi-square test. A p value under 0.05 is regarded as a statistically significant value.
Results
Sixty-two randomly selected patients diagnosed with squamous cell carcinoma who underwent surgery in our clinic for laryngeal cancer were included in this study. The study group included 1 female patient and 61 male patients. Of this total, 47 (75.8%) have a history of smoking. The youngest patient was 44 years old, and the oldest was 84 years old. The average age of the test group is 62.70.
One of the patients (1.6%) was designated as T1, 23 (37%) as T2, 12 (19.3%) as T3, 27 (43.5%) as T4, 40 as (64.5%) N0, 17 as (27.4%) N1 and 5 as (8%) N2. For statistical analysis, 5 N2 patients were included in the N1 group. Also, because two patients’ tumor differentiations were negligible, these patients were also included in the medium differentiate group.
36 (58%) patients had transglottic cancer, 18 (29%) patients had supraglottic cancer and 8(13%) patients had subglottic cancer. Thirtyfive patients (56.6%) underwent total laryngectomies, 20 patients (32.2%) had horizontal laryngectomies, 7 patients (11.2%) had vertical partial laryngectomies, 58 (93.5%) patients underwent neck dissections, 26 (41%) patients received postoperative radiotherapy, 11 (17%) patients received postoperative concomitance, and 2 (3%) patients received postoperative KT. The analysis of FHIT expression revealed that out of 62 primary tumor patients, FHIT expression disappeared in 39 (62.9%) patients, and was normal in 23 (37.1%) patients. The analysis of WWOX expression revealed that out of 62 primary tumor patients, WWOX expression disappeared in 29 (46.8%) patients and was normal 33 (53.2%) patients.
Expression values of FHIT and WWOX proteins, of which losses were parallel, were found to be statistically significant (chi-square p<0.012) (Table 1).
| WWOX (-) | WWOX (+) | TOTAL | |
|---|---|---|---|
| FHIT (-) | 23(%59) | 16(%41) | 39 |
| FHIT (+) | 6(26.1) | 17(73.9) | 23 |
| TOTAL | 29(%46.8) | 33(%53.2) | 62 |
Table 1: Expression of FHIT and WWOX proteins.
FHIT expression in tumor tissues
In the analysis of the T stage of tumor and distribution of FHIT expression, it was detected that different T phases varied in a statistically significant manner with respect to FHIT expression. As the T stage of the tumor increased, FHIT expression decreased (p=0.04) (Table 2).
| T stage | FHIT (-) | FHIT (+) | TOTAL |
|---|---|---|---|
| 1 | 3 (%7.69) | 1(%4.4) | 4(%6.45) |
| 2 | 4 (%10.2) | 9 (%39.1) | 13(%20.9) |
| 3 | 10 (%25.7) | 6 (%26.1) | 16(%25.8) |
| 4 | 22 (%56.41) | 7 (%30.4) | 29(%46.77) |
| TOTAL | 39 | 23 | 62 |
Table 2: T stage and expression of FHIT.
A statistically significant difference was detected in the volume of the tumor and FHIT expression distribution. The higher tumor diameter indicated a lower FHIT expression (p<0.05) (Table 3).
| Volume | FHIT (-) | FHIT (+) | TOTAL |
|---|---|---|---|
| 2 VE <2 | 19(%52.8) | 17(%47.2) | 36 |
| >2 | 20(%76.9) | 6(%23.1) | 26 |
| TOTAL | 39(%62.9) | 23(%37.1) | 62 |
Table 3: Volume of tumor and FHIT expression.
Forty one (66.1%) patients in the test group had a history of smoking in their anamneses. When the distribution of FHIT expression for these patients was analyzed, the expression of FHIT disappeared, leading to the conclusion that was statistically significant (p=0.006) (Table 4).
| SMOKING | FHIT (-) | FHIT (+) | TOTAL |
|---|---|---|---|
| Smoking (-) | 8(%38.1) | 13(%61.9) | 21 |
| Smoking (+) | 31(%75.6) | 10(%24.4) | 41 |
| TOTAL | 39(%62.9) | 23(%37.1) | 62 |
Table 4: Smoking history and FHIT expression.
An analysis of the relationship between the tumor’s lymph node metastasis and FHIT expression distribution in the primary tumor revealed no metastatic lymph node in patients with decreased FHIT expression, and this was statistically significant (p=0.012) (Table 5).
| LYMPH NODE | FHIT (-) | FHIT (+) | TOTAL |
|---|---|---|---|
| Metastasis (-) | 30(%75) | 10 (%25) | 40 |
| Metastasis (+) | 9 (%40.9) | 13 (%59.1) | 22 |
| TOTAL | 39 (%62.9) | 23 (%37.1) | 62 |
Table 5: Lymph node metastasis and FHIT expression.
No statistically significant difference was among the factors: tumor location, presence of perineural invasion, surgical limit positivity, tumor differentiation, cartilage invasion, treatment or non-treatment with postoperative radiotherapy, tumor location, presence of distant metastasis, patient follow-up and distribution of FHIT expression. Regardless of the absence of statistically significant differences, in 20 (74.1%) patients with cartilage invasion, FHIT expression disappeared, which was important.
When age and FHIT expression were compared in 35 patients (56.5%) below the age of 60, and 27 patients (66.7%) above the age of 60 years, FHIT expression disappeared, but this finding is not statistically significant (p=0.587).
WWOX expression in tumor tissues
When the distribution of WWOX expression in the primary tumor was analyzed, different phases of T varied statistically with respect to WWOX expression (p=0.036). As the T stage of the tumor increased, WWOX expression decreased (Table 6).
| T stage | WWOX (-) | WWOX (+) | TOTAL |
|---|---|---|---|
| 1 | 1 (%25) | 3(%75) | 4 |
| 2 | 2(%15.4) | 11(%84.6) | 13 |
| 3 | 9(%56.3) | 7(%43.8) | 16 |
| 4 | 17(%58.6) | 12(%41.4) | 29 |
| TOTAL | 29(%46.8) | 33(%53.2) | 62 |
Table 6: T stage and WWOX expression.
There was a statistically significant difference between distant metastasis and the distribution of WWOX expression (p=0.043). As distant metastasis increased, WWOX expression decreased (Table 7).
| Distance metastasis | WWOX (-) | WWOX (+) | TOTAL |
|---|---|---|---|
| Metastasis (-) | 25(%43.1) | 33(%56.9) | 58 |
| Metastasis (+) | 4(%100) | 0(%0) | 4 |
| TOTAL | 29(%46.8) | 33(%53.2) | 62 |
Table 7: Distance metastasis and WWOX expression.
When the distribution of WWOX expression in primary tumors are compared with the presence of metastatic lymph nodes, no statistically significant difference could be detected (p=0.5), yet in 13 patients (59.1%) with metastatic lymph nodes, WWOX expression did not disappear.
No statistically important relationship was found between WWOX expression and tumor location, perineural invasion, surgical limit, tumor differentiation, tumor location, smoking history, postoperative supplementary treatment, cartilage invasion, or patient follow-up.
Regarding smoking history, though not statistically important, 13 (61.9%) non-smoker patients and 20 (48.8%) smoker patients experienced WWOX expression.
When tumor volume was analyzed, in 15 patients (41.7%) with a tumor volume smaller than 2 cm and in 14 patients (53.8%) with tumor volume larger than 2 cm, WWOX expression disappeared.
WWOX expression disappeared in 29 patients (47.8%) below the age of 60, and in 33 patients (46.2%) above the age of 60 (p=1). These values are not statistically significant.
The relationship between FHIT and WWOX expressions with a remission period were explored via the Kaplan-Meier life analysis test. Remission periods are shorter in patients with decreasing FHIT and WWOX expression, but this relationship is not statistically important.
Discussion
Laryngeal cancer with squamous cells is a frequent, clinically aggressive, malignant tumor among cancers of the head and neck, and can progress quickly [7]. In order to better envisage clinical results of the disease, apply optimal treatment protocols, and pre-decide adjuvant treatment in laryngeal cancers, there are various prognostic factors available. Tumor size and the presence of cervical metastatic lymph nodes, which detects the invasion and metastatic potential for tumor growth, are amongst the most critical prognostic factors. Hence it has been suggested that if reliable parameters that indicate the invasion and metastatic potential for tumor growth are detected, prognosis of the disease and treatment protocols for patients can be better determined [8-10]. In some studies; PCNA [11], nm23 [12] are more effective than histological things in determining the prognosis. They have also reported that in lower lip and laryngeal cancers, tumor thickness is also a prognostic factor [13]. Furthermore, in laryngeal cancer it has been determined that Langerhans cell infiltration has a meaningful correlation with other prognostic parameters; hence it could be a prognostic factor [14].
A number of oncogenes and tumor suppressor genes have been investigated for carcinogenesis. Driven from the fact that carcinogens are effective in certain regions, many studies have been conducted on FRA3B (3p14.2), which is one of the most fragile sections of the human chromosome. It has been deduced that FHIT and WWOX genes are effective as tumor suppressor genes in carcinogenesis. Therefore, since FHIT and WWOX gene expression can be a prognostic factor in various tumors, they have become the focus of further research.
Studies related to the loss of FHIT expression emerged after 1996. Loss of FHIT expression has been detected as a negative prognosis marker for a good number of cancer types, with the most common types being cancers of the lung, colon, gastrointestinal system, and tongue [15-20].
Another researcher has pointed to the fact that in lung precancerous lesions, loss of FHIT is more frequent in patients with a history of smoking [21].
When analyzing studies relevant to head and neck cancers, a loss of FHIT expression is a negative prognosis factor in patients with head and neck cancer and that in patients with a loss of FHIT expression life span decreases by five years. It has been reported that the risk of distant metastasis is higher, but not statistically significant, yet as loco regional recurrence is cross-referenced, it was found to be rare in patients with FHIT expression loss. It has been argued that in patients with FHIT expression loss, sensitivity to radiotherapy is higher, hence loco regional recurrence is lower [22]. It has thus been concluded that the tumors in patients with decreasing FHIT expression are radiosensitive. It has also been reported that there is not a relationship between clinical and pathologic features of patients with FHIT expression.
Other research has indicated that there is no relationship between FHIT expression and the clinical correlation of head and neck cancers (histological grade, tumor stage, alcohol-tobacco and even prognosis) [23-25].
In another study it was stated that FHIT expression is related to the prognosis of esophageal cancer, but not related to patient prognosis and tobacco or alcohol consumption. The researchers have also demonstrated that decreased FHIT expression is related to tumor infiltration and metastatic lymph node carcinoma. They have also determined that in metastatic lymph node carcinoma, FHIT expression has no relationship to primary tumor expression [26]. Pavelic et al. [27] have stated that in malignant thyroid gland lesions, the decreasing FHIT expression and apoptosis can be related to malignance and be used as a prognostic factor.
In this study, FHIT expression in 39 patients (62.9%) disappeared. FHIT expression disappeared while the stage increased. A statistically significant relationship was observed between tumor diameter and FHIT expression, where in a higher tumor volume indicated a lower FHIT expression. Additionally it was observed that in 70% of patients with distant metastases, there were advanced levels of FHIT expression loss.
An analysis of the relevant literature shows that in head and neck cancers, smoking does not affect FHIT expression, but the present study demonstrates smoking and FHIT expression are interrelated, and in patients who smoke, FHIT expression decreases to a great extent. As lymph node metastasis was analyzed, the envisaged correlation does not exist between lymph node metastases and primary tumor FHIT expression; a greater presence of metastases indicates a higher rate of FHIT expression. Certain studies in literature also point out that in lung cancer there have been seen similar results between metastatic lymph node and FHIT expression. Though not statistically significant, in 54.3% of patients with cartilage invasion and 61.5% of patients with distant metastases, there was loss of FHIT expression.
The WWOX gene is a tumor suppressor gene expressed on the 16q23.3-24.1 chromosomes in the FRA16D region [28]. It is also known as WWOX FOR2. Loss of expression plays role in the emergence of cancer of the breast, ovary, prostate, esophagus, lungs, and pancreas [29-31]. Consequently, it has been established that WWOX is a tumor suppressor gene. The region in which the WWOX gene is located, as well as its inactivation pattern, is rather similar to those of the FHIT gene.
Aqeilan et al. [31] have explained in their research that there is a relationship between WWOX expression and gastric adenocancer histological grade. In this study, as well, the meaningful relationship between FHIT and WWOX expression has been outlined and a statistically meaningful correlation between both protein expressions has been verified.
Other studies have indicated that in esophagus and lung cancer there is a deletion or transformation in the WWOX gene [29-32]. It has also been reported that in non-small-cell lung cancer [33], esophagus cancer [30], and breast cancer [34], that the WWOX gene inactivation plays a role. There are also some studies showing that regional genetic changes expressing this gene are environmental causes (UV lights and environmental carcinogens) [35]. It has also been reported that alcohol and tobacco consumption are effective in the emergence of esophageal and lung cancer due to the transformation they create in the FRA16D and FRA3B chromosomal regions [36-40].
It has been underlined in another research that decreasing WWOX expression is effective in the development of thyroid cancer, as well [41].
In a different study, the relationship between oral leukoplakia and WWOX expression was examined. In 35% of the analyzed cases, there was a transformation in mRNA transcription or a decrease in WWOX expression [42]. Since transformations in the WWOX gene may be indicative of the early phase of oral cavity cancers, they may be influential in the commencement of early treatment.
In our research, a statistically significant correlation was established between decreasing WWOX protein expression and distant metastasis and the tumor stage. As distant metastasis increased, or the tumor stage progressed, WWOX expression decreased greatly. An unusual correlation was also observed between lymph node metastasis and gene expression. In a majority of metastasis patients the expression has not disappeared, but this is not statistically important. No important relationship could be seen between changes in WWOX expression and smoking history, perineural invasion, surgical limit, tumor diameter, postoperative treatment, or tumor location.
Considering that as T stage of a tumor increases, expressions of the FHIT and WWOX proteins decrease greatly, and as the tumor volume increases, FHIT expression conversely decreases. As distant metastasis increases, WWOX expression decreases are all indicative that these proteins may be effective in laryngeal cancer. Tumor phase and diameter, presence of distance metastasis are factors indicating the prognosis of the disease; the prognosis of the disease gets worse as the phase and diameter increases and distant metastasis is present. It is significant that these proteins and these prognostic factors are correlated, yet no statistically significant relationship could be detected with a remission period. However, the results demonstrate that as protein expression decreases, the remission period is shortened. The lack of a statistically significant relationship has been attributed to the limited number of the patients examined herein. Furthermore, a correlation was been found between smoking (of which the effect in laryngeal cancer was already proven) and FHIT expression. It was witnessed that in patients who smoke, FHIT expression decreased, which points to the possibility that tobacco can be responsible for cancer due to the changes it creates in the human genome.
References
- Blackwell KE, Calcaterra TC, Fu YS (1995) Laryngeal dysplasia: epidemiology and treatment outcome. Ann Otol Rhinol Laryngol 104: 596-602.
- Yilmaz T, Hosal AS, Gedikoglu G, Kaya S (1999) Prognostic significance of histopathological parameters in cancer of the larynx. Eur Arch Otorhinolaryngol 256: 139-44.
- Spafford MF, Koeppe J, Pan Z, Archer PG, Meyers AD, et al. (1996) Correlation of tumor markers p53, bcl-2, CD34, CD44H, CD44v6, and Ki-67 with survival and metastasis in laryngeal squamous cell carcinoma. Arch Otolaryngol Head Neck Surg 122: 627-632.
- Kupisz K, Chibowski D, Klatka J, Klonowski S, Stepulak A (1999) Tumor angiogenesis in patients with laryngeal cancer. Eur Arch Otorhinolaryngol 256: 303-305.
- Dumon KR, Ishii H, Vecchione A, Trapasso F, Baldassarre G, et al. (2001) Fragile histidine triad expression delays tumor development and induces apoptosis in human pancreatic cancer. Cancer Res 61: 4827-4836.
- Darai E, Bringuier AF, Walker-Combrouze F, Fauconnier A, Couvelard A, et al. (1998) CD31 expression in benign, borderline, and malignant epithelial ovarian tumors: an immunohistochemical and serological analysis. Gynecol Oncol 71: 122-127.
- Cazorla M, Hernández L, Nadal A, BalbÃÂn M, López JM, et al. (1998) Collagenase-3 expression is associated with advanced local invasion in human squamous cell carcinomas of the larynx. J Pathol 186: 144-150.
- Hong SD, Hong SP, Lee J, Lim CY (2000) Expression of matrix metalloproteinase-2 and 9 in oral squamous cell carcinomas with regard to the metastatic potential. Oral Oncol 36: 207-213.
- Imanishi Y, Fujii M, Tokumaru Y, Tomita T, Kanke M, et al. (2000) Clinical significance of expression of Membrane type 1 matrix metalloproteinase-2 in human head and neck squamous cell carcinoma. Hum Pathol 31: 895-904.
- Kurahara S, Shinohara M, Ikebe T, Nakamura S, Beppu M, et al. (1999) Expression of MMPs, MTMMP and TIMPs in squamous cell carcinoma of the oral cavity correlations with tumor invasion and metastasis. Head and Neck 21: 627-638.
- Saraç S, Ayhan A, Hosal AS, Kaya S (1998) Prognostic significance of PCNA expression in laryngeal cancer. Arch Otolaryngol Head Neck Surg 124: 1321-1324.
- Gunduz M, Ayhan A, Gullu I, Onerci M, Hosal AS, et al. (1997) nm23 Protein expression in larynx cancer and the relationship with metastasis. Eur J Cancer 33: 2338-2341.
- Önerci M, Yilmaz T, Gedikoglu G (2000) Tumor thickness as a predictor of cervical lymph node metastasis in squamous cell carcinoma of the lower lip. Otolaryngol Head Neck Surg 122: 139-142.
- Yilmaz T, Gedikoglu G, Celik A, Onerci M, Turan E (2005) Prognostic significance of Langerhans cell infiltration in cancer of the larynx. Otolaryngol Head Neck Surg 132: 309-316.
- Tomizawa Y, Nakajima T, Kohno T, Saito R, Yamaguchi N, et al. (1998) Clinicopathological significance of Fhit protein expression in stage I non-small cell lung carcinoma. Cancer Res 58: 5478-5483.
- Mady HH, Melhem MF (2002) FHIT protein expression and its relation to apoptosis, tumor histologic grade and prognosis in colorectal adenocarcinoma: an immunohistochemical and image analysis study. Clin Exp Metastasis 19: 351-358.
- Capuzzi D, Santoro E, Hauck WW, Kovatich AJ, Rosato FE, et al. (2000) Fhit expression in gastric adenocarcinoma: correlation with disease stage and survival. Cancer 88: 24-34.
- Lee JI, Soria JC, Hassan K, Liu D, Tang X, et al. (2001) Loss of Fhit expression is a predictor of poor outcome in tongue cancer. Cancer Res 61: 837-841.
- Sozzi G, Tornielli S, Tagliabue E, Sard L, Pezzella F, et al. (1997) Absence of Fhit protein in primary lung tumors and cell lines with FHIT gene abnormalities. Cancer Res 57: 5207-5212.
- Greenspan DL, Connolly DC, Wu R, Lei RY, Vogelstein JT, et al. (1997) Loss of FHIT expression in cervical carcinoma cell lines and primary tumors. Cancer Res 57: 4692-4698.
- Sozzi G, Pastorino U, Moiraghi L, Tagliabue E, Pezzella F, et al. (1998) Loss of FHIT function in lung cancer and preinvasive bronchial lesions. Cancer Res 58: 5032-5037.
- Tai SK, Lee JI, Ang KK, El-Naggar AK, Hassan KA, et al. (2004) Loss of Fhit expression in head and neck squamous cell carcinoma and its potential clinical implication. Clin Cancer Res 10: 5554-5557.
- Mineta H, Miura K, Takebayashi S, Misawa K, Ueda Y, et al. (2003) Low expression of fragile histidine triad gene correlates with high proliferation in head and neck squamous cell carcinoma. Oral Oncol 39: 56-63.
- Paradiso A, Ranieri G, Stea B, Zito A, Zehbe I, et al. (2004) Altered p16INK4a and Fhit expression in carcinogenesis and progression of human oral cancer. Int J Oncol 24: 249-255.
- Guerin LA, Hoffman HT, Zimmerman MB, Robinson RA (2006) Decreased fragile histidine triad gene protein expression is associated with worse prognosis in oral squamous carcinoma. Arch Pathol Lab Med 130: 158-164.
- Shimada Y, Sato F, Watanabe G, Yamasaki S, Kato M, et al. (2000) Loss of FHIT gene expression is associated withprogression of esophageal squamous cell carcinoma, but not with the patient’s prognosis and smoking history. Cancer 89: 5-11.
- Pavelić K, Dedivitis RA, Kapitanović S, Cacev T, Guirado CR, et al. (2006) Molecular genetic alterations of FHIT and p53 genes in benign and malignant thyroid gland lesions. Mutat Res 599: 45-57.
- Bednarek AK, Laflin KJ, Daniel RL, Liao Q, Hawkins KA, et al. (2000) Wwox, a novel ww domain –containing protein mapping to human chromosome 16q23.3-24.1, a region frequently affected in breast cancer. Cancer Res 60: 2140-2145.
- Kuroki T, Trapasso F, Shiraishi T, Alder H, Mimori K, et al. (2002) Genetic alterations of the tumor suppressor gene WWOX in esophageal squamous cell carcinoma. Cancer Res 62: 2258-2260.
- Paige AJ, Taylor KJ, Taylor C, Hillier SG, Farrington S, et al. (2001) WWOX: a candidate tumor suppressor gene involved in multiple tumor types. Proc Natl Acad Sci U S A 98: 11417-11422.
- Aqeilan RI, Kuroki T, Pekarsky Y, Albagha O, Trapasso F, et al. (2004) Loss of WWOX expression in gastric carcinoma. Clin Cancer Res 10: 3053-3058.
- Yendamuri S, Kuroki T, Trapasso F, Henry AC, Dumon KR, et al. (2003) WW domain containing oxidoreductase gene expression is altered in non-small cell lung cancer. Cancer Res 63: 878-881.
- Ludes-Meyers JH, Bednarek AK, Popescu NC, Bedford M, Aldaz CM (2003) WWOX, the common chromosomal fragile site, FRA16D, cancer gene. Cytogenet Genome Res 100: 101-110.
- Paige AJ, Taylor KJ, Stewart A, Sqouros JG, Gabra H, et al. (2000) A 700-kb physical map of a Region of 16q23.2 homozygously deleted in multiple cancers and spanning the common fragile site FRA16D. Cancer Res 60: 1690-1697.
- O'Keefe LV, Richards RI (2006) Common chromosomal fragile sites and cancer: focus on FRA16D. Cancer Lett 232: 37-47.
- Sozzi G, Sard L, De Gregorio L, Marchetti A, Musso K, et al. (1997) Association between cigarette smoking and FHIT gene alterations in lung cancer. Cancer Res 57: 2121-2123.
- Mori M, Mimori K, Shiraishi T, Alder H, Inoue H, et al. (2000) Altered expression of fhit in carcinoma and precarcinomatous lesions of the esophagus. Cancer Res 60: 1177-1182.
- Stein CK, Glover TW, Palmer JL, Glisson BS (2002) Direct correlation between FRA3B expression and cigarette smoking. Genes Chromosomes Cancer 34: 333-340.
- Lai FJ, Cheng CL, Chen ST, Wu CH, Hsu LJ, et al. (2005) WOX1 is essential for UVB irradiation-induced apoptosis and down-regulated via translational blockade in UVB-induced cutaneous squamous cell carcinoma in vivo. Clin Cancer Res 11: 5769-5777.
- Pimenta FJ, Gomes DA, Perdigão PF, Barbosa AA, Romano-Silva MA, et al. (2006) Characterization of the tumor suppressor gene WWOX in primary human oral squamous cell carcinomas. Int J Cancer 118: 1154-1158.
- Dias EP, Pimenta FJ, Sarquis MS, Dias Filho MA, Aldaz CM, et al. (2007) Association between decreased WWOX protein expression and thyroid cancer development. Thyroid 17: 1055-1059.
- Pimenta FJ, Cordeiro GT, Pimenta LG, Viana MB, Lopes J, et al. (2008) Molecular alterations in the tumor suppressor gene WWOX in oral leukoplakias. Oral Oncol 44: 753-758.
Citation: Çağlar O, Tezel G, Ruaçan A, Sözeri B (2013) Prognostic Significance of Fhit and Wwox Proteins Expression in Laryngeal Cancer. Otolaryngology 3:148. DOI: 10.4172/2161-119X.1000148
Copyright: © 2013 Çağlar O, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 15018
- [From(publication date): 11-2013 - Apr 05, 2025]
- Breakdown by view type
- HTML page views: 10164
- PDF downloads: 4854