Observation of Viable Nontypeable Haemophilus Influenzae Bacteria within Neutrophil Extracellular Traps in Clinical Samples from Chronic Otitis Media
Received: 06-Sep-2013 / Accepted Date: 01-Oct-2013 / Published Date: 09-Oct-2013 DOI: 10.4172/2161-119X.1000145
Abstract
Bacterial otitis media is an inherently inflammatory condition and often features an influx of neutrophils and other phagocytes into the middle-ear chamber. Neutrophils undergo a specific death pathway that results in formation of Neutrophil Extracellular Traps or “NETs”, which have been postulated as an antimicrobial defense mechanism that both mediates direct bacterial killing and facilitates phagocytic uptake and killing. However, we have shown that some mucosal pathogens within the airway including nontypeable Haemophilus influenzae survive within NETs in animal and in vitro models. In this report, we utilize exudate samples obtained from patients with chronic otitis media to show that nontypeable H. influenzae bacteria survive within NET structures within human patients.
Keywords: Neutrophil, Exudate, Bacteria, Otitis media
255034Introduction
Otitis Media (OM) is an extremely common and costly pediatric illness, with an economic burden exceeding $3 billion per year in the U.S. alone [1]. OM is caused by dysfunction of the Eustachian tube, which facilitates persistent colonization of the middle-ear chamber by bacterial opportunists that normally reside within the nasopharyngeal microbiota [2-8]. These bacteria persist within multicellular biofilm communities that afford inherent resistance to clearance [9-18]. Recent work has demonstrated that bacterial biofilms observed in the chinchilla infection model for otitis media contain a significant proportion of host extracellular DNA [19], and fit many of the defining characteristics of neutrophil extracellular traps or NETs [20] which have been proposed to mediate direct killing and augment phagocytic clearance of bacteria and other microbes [21,22]. However, work from our laboratory and others using in vitro models have shown that NETs are not an efficient killing mechanism for major otopathogenic bacterial strains, and instead that nontypeable H. influenzae and encapsulated pneumococci survive within the NET structure [20,23,24]. In this study, inflammatory exudate material was recovered from the middle-ear chamber of a patient undergoing tympanostomy surgery for chronic/ recurrent OM infections and used to address the hypotheses that: i) neutrophil extracellular traps occur in human patients and ii) as in our model studies, they lack the ability to resolve bacterial infection.
Methods
Patient samples
Samples of middle-ear effusion/exudate were obtained as discard tissue from children undergoing tympanostomy surgery for chronic/ recurrent otitis media at Wake Forest Baptist Hospital. The samples were collected as a blinded set with no patient identifiers. The Wake Forest Internal Review Board reviewed and approved this work as an exempt study using discard tissue samples.
Isolation and cryosectioning of biofilms
Solid material identified as resembling biofilms in middle ear effusions was collected from patient samples. For cryosection, the samples were placed into Cryomolds, Tissue-Tek OCT compound (Sakura Finetek, CA) was added, and the blocks were frozen at -80°C. Serial 5-μm sections were cut using a cryotome at -20°C and stored at -80°C prior to further analyses.
Microscopic detection of biofilms
For immunofluorescent staining, the slides were brought to room temperature and fixed briefly with 2% paraformaldehyde/phosphate buffered saline solution (pH=7.5) prior to antibody and DNA staining. To detect nontypeable H. influenzae bacteria present within biofilms, individual sections were stained with rabbit antisera directed against H. influenzae, followed with an Alexa-488 donkey anti-rabbit secondary antibody [20]. For detection of histones, sections were stained with mouse anti-histone (monoclonal antibody H11-4/ MAB3422; Chemicon) followed by Alexa 488 goat anti-mouse secondary antibody. In some sections, propidium iodide was used to label DNA. Samples were analyzed for immunofluorescence using a Nikon Eclipse C1 Confocal Laser Scanning Microscope (CLSM).
Histopathology
For histological examination, sections were stained with Hematoxylin and Eosin (H&E) according to standard methodology, and viewed on a Nikon Eclipse microscope [25].
Bacterial strains used in in-vitro assays
Nasopharyngeal isolate and commonly used lab strain 86-028NP was used in in-vitro assays along with clinical OM isolate OTIS-2. OTIS-2 was isolated from middle ear effusions collected as a result of routine tympanostomy tube placement [8]. Bacterial strains were stored at -80°c in S-BHI broth (brain heart infusion media supplemented with NAD and Hemin) with 20% glycerol. The strains were plated on S-BHI agar and grown overnight at 37°C after which they were harvested and suspended in S-BHI broth to an OD600 corresponding to approximately 108 colony-forming units/ml.
In vitro NET survival assay
Human peripheral neutrophils were collected from healthy volunteers via peripheral blood draw and purified via Isolymph density-gradient centrifugation. Glass coverslips were coated with poly-L-lysine (Sigma-Aldrich) and cells were allowed to adhere. As described previously [23], in some samples NET formation was induced by treatment with heat-inactivated 86-028NP, and in some assays cells were pretreated with cytochalasin D (Sigma-Aldrich) for ten minutes at 37°C to inhibit phagocytosis. Bacteria were added to coverslips at an MOI of 10:1 and incubated at 37°C for one hour. Neutrophils were then lysed with 0.05% saponin and bacteria enumerated via serial dilution and plate count.
Results
Immunofluorescent staining
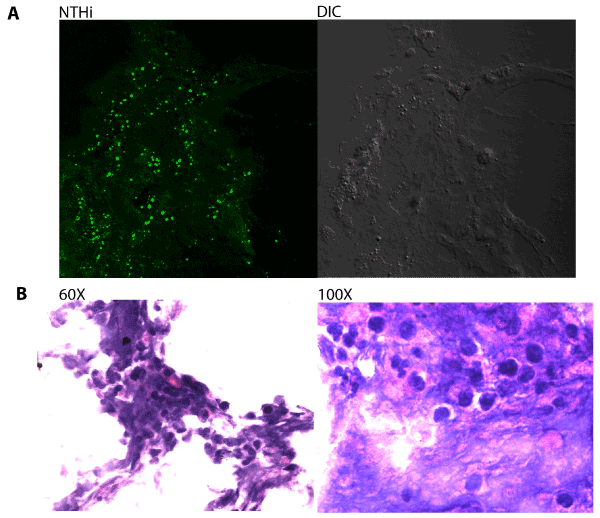
Immunofluorescent staining was first used to visualize the presence of Nontypeable H. influenzae (NTHi) bacteria within host-derived biofilm material obtained from middle-ear effusion samples. Biofilms were cryosectioned, fixed and stained with rabbit antisera directed against H. influenzae. NTHi bacteria were frequently observed within the sections (Figure 1A), and staining of adjacent sections with Hektoin/ Eosin methylene blue revealed significant infiltration of neutrophils and other phagocytic host cells (Figure 1B).
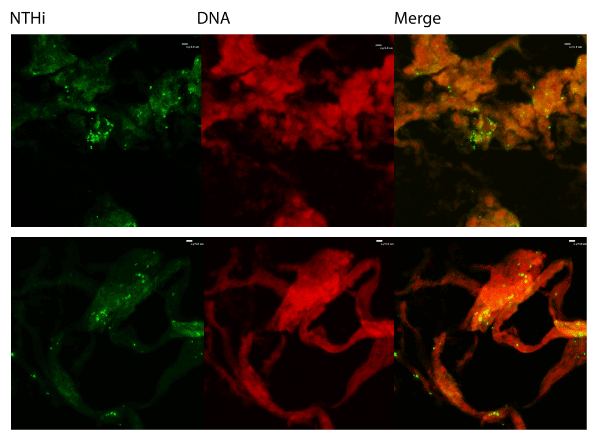
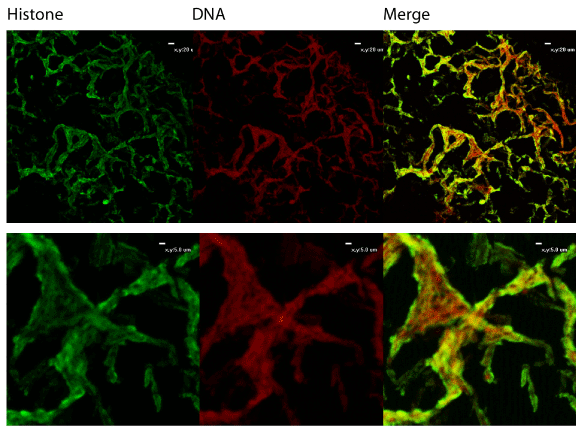
We also utilized immunofluorescent staining to assess presence of Neutrophil Extracellular Trap (NET) structures within the middle ear during OM infection. The first indication that NETs are present is that NTHi appear to be embedded in a host-derived DNA matrix (Figure 2). This is a hallmark of NETs and is an innate immune defense which has been postulated to entrap microbes and both mediate direct killing as well as facilitate uptake and phagocytic killing by incoming neutrophils. The typical NET structure features a double-stranded DNA lattice decorated with histones and granular components [22]. We examined the biofilms further to determine if this DNA was part of a larger NET structure. Staining showed colocalization of host DNA and histones, suggesting that these are host-produced NETs (Figure 3).
Figure 2: Immunofluorescent staining. Biofilms obtained from middleear effusions were cryosectioned and stained with rabbit antisera against H. influenzae or propidium iodide to show that NTHi are embedded in a DNA matrix. Sections were analyzed for immunofluorescence using a Nikon Eclipse C1 confocal laser scanning microscope.
In vitro survival assay
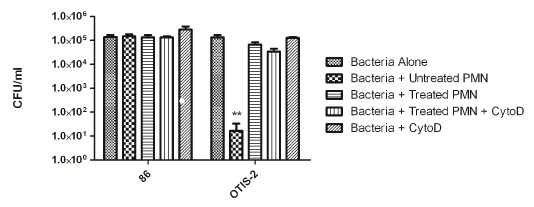
We have previously shown that NTHi survive within NETs both in vitro and in an animal model of infection [20,23]. In order to determine if that was the case for these clinically derived strains, we used an in vitro survival assay. In this assay, neutrophils were isolated from healthy donors and adhered to a glass coverslip. NET formation was induced by treatment with heat-killed NTHi [23], and bacteria were added at an MOI of 10:1. For this assay, we used a well-characterized nasopharyngeal isolate, 86- 028NP, as well as one of the newly isolated OM isolates, OTIS-2. Some neutrophils were treated with cytochalasin D to inhibit phagocytosis, so these controls showed purely NET killing, whereas untreated neutrophils could kill by either phagocytosis or NETs. As previously shown, 86- 028NP is resistant to killing by neutrophils (Figure 4) [23]. OM strain OTIS-2 is killed by intact neutrophils via the phagocytic pathway, but it is not killed when NET formation is induced (Figure 4).
Figure 4: Clinical isolates of NTHi survive killing by NETs in vitro. Human neutrophils were adhered to glass slides and then treated with heat-inactivated H. influenzae to initiate NET-formation, cytochalasin D to inhibit phagocytosis, or left untreated to examine survival via the phagocytic pathway. Control wells contained bacteria alone with no added neutrophils. The results are from three independent experiments, each done in triplicate. Bars indicate standard error of the mean. **P < 0.05 compared with other variables.
Discussion
Otitis media is, by its very nature, an inflammatory condition that features a prominent phagocytic component. Neutrophils are the first-responders to a site of infection and typically ingest and kill bacteria. In addition, neutrophils are capable of eliminating pathogens extracellularly through the formation of NETs. Recent data from our laboratory and others have shown that the pathogens associated with this disease can survive and proliferate within neutrophil extracellular traps in the middle-ear chamber [20,23,26]. The results presented herein validate these findings in a clinical setting, as structures consistent with NET interlaced with NTHi bacteria were observed in middleear exudate/effusion samples obtained from patients undergoing tympanostomy surgery for chronic/recurrent otitis media. Moreover, a direct clinical isolate (OTIS-2) was completely resistant to killing within an in vitro model for NET-mediating bactericidal activity, as we have previously reported for laboratory model NTHi strains [20,23].
As is true for most opportunists associated with OM infections, NTHi are able to persist for long periods of time within the middle-ear chamber and upper airways. Certainly, a greater understanding of the means for how these bacteria survive and replicate even in the face of the host immune defenses could easily afford insights and opportunities for better management both of the bacterial infections that drive OM and the host inflammatory response that is its hallmark.
Acknowledgements
The authors acknowledge assistance from Tina R. Swords in obtaining and documentation of clinical specimens. This work was supported by a grant from NIH/NIDCD (RO1DC007444). A.C.P. was supported by a training fellowship from the NIH (T32AI007401).
References
- Pichichero ME, Casey JR, Hoberman A, Schwartz R (2008) Pathogens causing recurrent and difficult-to-treat acute otitis media. Clin Pediatr (Phila) 47: 901-906.
- Pichichero ME (2005) Evolving shifts in otitis media pathogens: relevance to a managed care organization. Am J Manag Care 11: S192-201.
- Post JC, Preston RA, Aul JJ, Larkins-Pettigrew M, Rydquist-White J, et al. (1995) Molecular analysis of bacterial pathogens in otitis media with effusion. JAMA 273: 1598-1604.
- Pettigrew MM, Gent JF, Revai K, Patel JA, Chonmaitree T (2008) Microbial interactions during upper respiratory tract infections. Emerg Infect Dis 14: 1584-1591.
- Laufer AS, Metlay JP, Gent JF, Fennie KP, Kong Y, et al. (2011) Microbial communities of the upper respiratory tract and otitis media in children. MBio 2: e00245-10.
- Revai K, Mamidi D, Chonmaitree T (2008) Association of nasopharyngeal bacterial colonization during upper respiratory tract infection and the development of acute otitis media. Clin Infect Dis 46: e34-37.
- Holder RC, Kirse DJ, Evans AK, Peters TR, Poehling KA, et al. (2012) One third of middle ear effusions from children undergoing tympanostomy tube placement had multiple bacterial pathogens. BMC Pediatr 12: 87.
- Bakaletz LO (2012) Bacterial biofilms in the upper airway - evidence for role in pathology and implications for treatment of otitis media. Paediatr Respir Rev 13: 154-159.
- Bakaletz LO (2007) Bacterial biofilms in otitis media: evidence and relevance. Pediatr Infect Dis J 26: S17-9.
- Swords WE (2012) Nontypeable Haemophilus influenzae biofilms: evidence and relevance. Frontiers in Cell Infect Microbiol 2: 97.
- Hong W, Pang B, West-Barnette S, Swords WE (2007) Phosphorylcholine expression by nontypeable Haemophilus influenzae correlates with maturation of biofilm communities in vitro and in vivo. J Bacteriol 189: 8300-8307.
- Hong W, Mason K, Jurcisek JA, Novotny LA, Bakaletz LO, et al. (2007) Phosphorylcholine decreases early inflammation and promotes the establishment of stable biofilm communities of nontypeable Haemophilus influenzae strain 86-028NP in a chinchilla model of otitis media. Infect Immun 75: 958-965.
- Pang B, Hong W, Kock ND, Swords WE (2012) Dps promotes survival of nontypeable Haemophilus influenzae in biofilm communities in vitro and resistance to clearance in vivo. Frontiers in Cell and Infect Microbiol 2: 1-10.
- Reid SD, Hong W, Dew KE, Winn DR, Pang B et al. (2009) Streptococcus pneumoniae forms surface-attached communities in the middle ear of experimentally infected chinchillas. J Infect Dis 199: 786-794.
- Armbruster CE, Hong W, Pang B, Weimer KE, Juneau RA, et al. (2010) Indirect pathogenicity of Haemophilus influenzae and Moraxella catarrhalis in polymicrobial otitis media occurs via interspecies quorum signaling. MBio 1: 102-110.
- Armbruster C, Foster G, Learman B, Pang B, Swords WE (2013) LuxS expression promotes nontypeable Haemophilus influenzae biofilm development and prevents dispersal. Manuscript in review.
- Armbruster CE, Hong W, Pang B, Dew KE, Juneau RA, et al. (2009) LuxS promotes biofilm maturation and persistence of nontypeable Haemophilus influenzae in experimental otitis media by modulation of lipooligosaccharide composition. Infect Immun 77: 4081-4091.
- Jurcisek JA, Bakaletz LO (2007) Biofilms formed by nontypeable Haemophilus influenzae in vivo contain both double-stranded DNA and type IV pilin protein. J Bacteriol 189: 3868-3875.
- Hong W, Juneau R, Pang B, Swords WE (2009) Survival of bacterial biofilms within neutrophil extracellular traps promotes nontypeable Haemophilus influenzae persistence in the chinchilla model for otitis media. J Innate Immun 1: 215-224.
- Brinkmann V, Zychlinsky A (2007) Beneficial suicide: why neutrophils die to make NETs. Nat Rev Microbiol 5: 577-582.
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, et al. (2004) Neutrophil extracellular traps kill bacteria. Science 303: 1532-1535.
- Juneau RA, Pang B, Weimer KE, Armbruster CE, Swords WE (2011) Nontypeable Haemophilus influenzae initiates formation of neutrophil extracellular traps. Infect Immun 79: 431-438.
- Wartha F, Beiter K, Albiger B, Fernebro J, Zychlinsky A, et al. (2007) Capsule and D-alanylated lipoteichoic acids protect Streptococcus pneumoniae against neutrophil extracellular traps. Cell Microbiol 9: 1162-1171.
- Pang B, Winn D, Johnson R, Hong W, West-Barnette S, et al. (2008) Lipooligosaccharides containing phosphorylcholine delay pulmonary clearance of nontypeable Haemophilus influenzae Infect Immun 76: 2037-2043.
- Thornton RB, Wiertsema SP, Kirkham LA, Rigby PJ, Vijayasekaran S, et al. (2013) Neutrophil extracellular traps and bacterial biofilms in middle ear effusion of children with recurrent acute otitis media--a potential treatment target. PLoS ONE 8: e53837.
Citation: King LB, Pang B, Perez AC, Reimche JL, Kirse DJ, et al. (2013) Observation of Viable Nontypeable Haemophilus Influenzae Bacteria within Neutrophil Extracellular Traps in Clinical Samples from Chronic Otitis Media. Otolaryngology 3:145. DOI: 10.4172/2161-119X.1000145
Copyright: © 2013 King LB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 15614
- [From(publication date): 11-2013 - Apr 07, 2025]
- Breakdown by view type
- HTML page views: 10938
- PDF downloads: 4676