Cetuximab and Oral Mucositis: Is it Different from Oral Mucositis Caused by Other Drugs?
Received: 09-Oct-2013 / Accepted Date: 09-Nov-2013 / Published Date: 18-Nov-2013 DOI: 10.4172/2161-119X.1000147
Abstract
Purpose: To estimate frequency of oral mucositis in treatment for head and neck cancer with radiotherapy and concurrent cetuximab and to determine whether it has different characteristics from mucositis caused by other drugs.
Materials and methods: Subjects with locally advanced, primary non-metastatic, squamous cell carcinoma located in the oropharynx were treated with radiation therapy plus concomitant cetuximab. Results: None of patients received their full planned course of combination treatment due to excessive mucosal toxicity. All patients developed oral mucositis within about 10-15 days: it began directly, without escalation, as grade ≥ 3; it was associated with severe pain and trismus; it was never associated to specific supra-infections; the evolution and the consequent resolution of clinical discomfort required several days and the treatment with corticosteroids did not represent the solution formula.
Conclusion: Cetuximab induced oral mucositis have the following specific characteristics: time of onset, mode of clinical expression, severity, association with trismus and minimal response to corticosteroid therapy. Considering that the majority of studies do not reveal oral toxicity associated with cetuximab, future clinical trials should focus on specific topics to improve the definition of documented oral toxic effects.
Keywords: Cetuximab, Chemotherapy, Oral mucositis, Drugs
255117Introduction
Oral mucositis represents a common complication of nonsurgical treatment for head and neck cancer. It is associated with pain, dysgeusia and dysphagia, compromising nutritional status: therefore oral mucositis has a significant impact on patient’s quality of life. Its appearance depends on radiation and/or cytotoxic regimen adopted and on subjective-related factors, such as age, patient’s diagnosis and pre-treatment oral condition [1]. Oral side effects can induce a radiation total dose reduction or a treatment schedule modification; severe oral mucositis, consequently, can determine a discontinued treatment, to allow resolution of toxicity [2]. The evaluation of oral mucositis is paramount to compare different treatment efficacy, in term of survival too, because treatment disruptions can impact on local disease control. We analysed data on our head and neck database to estimate frequency and graveness of oral mucositis in patients treated with radiotherapy and concurrent cetuximab and to determine whether it has different characteristics from mucositis caused by other drugs.
Patients and Methods
Subjects with locally advanced, primary non-metastatic, squamous cell carcinoma located in the oropharynx were included. Patients received a complete work-up including count blood cells and determination of serum electrolyte levels, nasopharyngolaryngoscopy with biopsy, CT and/or RM of the head and neck, chest CT and preventive dental care.
Cetuximab was administered once a week. Patients received the initial induction dose of 400 mg/m2 one week before radiation therapy, followed by 250 mg/m2/week until the end of the radiation therapy period. A prophylactic premedication with corticosteroids and antihistamines was performed to reduce infusion-related reactions. According to recommendation by the manufacturer cetuximab therapy was interrupted if severe reaction (≥ grade 3) occurred [3].
Two radiation schedules were used: conventional radiotherapy (70 Gy in 35 daily fractions, 5 days/week, over 7 weeks) and simultaneous integrated boost (68-52.7 Gy in 31 daily fractions, 5 days/week, over 6 weeks).
Toxicity was classified by type and grade according to NCI-CTC version 4.0 [4]. Clinical photographs were taken of grade ≥ 3 mucosal reactions.
Results
Patients’ characteristics
Between May 2011 and September 2012, 5 consecutive patients with locally advanced squamous cell carcinoma of the oropharynx were treated with radiation therapy plus concomitant cetuximab at our Department. Demographic and clinical characteristics of patients are listed in Table 1. All patients were males, with PS 0-1 and average age of 63.2 years (range 54-69). All tumours were classified as stage IVA because of pathological regional lymph nodes.
| Characteristics | n | % |
|---|---|---|
| Sex | ||
| Male | 5 | 100 |
| Female | 0 | |
| Age | ||
| average | 63.2 | |
| range | 54-69 | |
| Performance status | ||
| 0 | 3 | 60 |
| 1 | 2 | 40 |
| Histology | ||
| Squamous cell carcinoma | 5 | 100 |
| T-stage | ||
| 1-2 | 3 | 60 |
| 3-4 | 2 | 40 |
| N-stage | ||
| 2b | 3 | 60 |
| 2c | 2 | 40 |
| AJCC stage | ||
| IVA | 5 | 100 |
| Treatment | ||
| RT + cetuximab | 5 | 100 |
Table 1: Demographic and tumour characteristics of patients.
Treatment compliance
All patients completed the cetuximab induction phase and all patients began the concomitant treatment. None of patients received their full planned course of combination treatment due to excessive mucosal toxicity. Target therapy was definitively suspended in all patients: 1 patient tolerated only 2 cycles of cetuximab; 2 patients received 3 cycles and 2 patients 4 cycles of cetuximab. Radiation therapy was interrupted for 12 days (range 10-17) in 4 patients; only 1 patient received radiation total dose as current practice.
Acute toxicity
Toxicities reported included oral mucositis, dysphagia, pain and skin reactions.
All patients developed oral mucositis within about 10-15 days: it began directly, without escalation, as grade 3 in 4 patients and as grade 4 in 1 patient. This symptom was associated with severe pain and trismus in all cases.
Radiation-induced skin toxicity was recorded: it occurred as G4 in 2 patients and G3 in the other patients.
Symptomatic and altered eating/swallowing (G2) was recorded in 4 patients, while 1 patient presented a severe dysphagia (G3).
Discussion
Oral mucositis monitoring is a crucial point in the assessment of therapies. The introduction of target therapy generated several adverse events which are not well characterized by the National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.0 and its previous versions (NCI-CTCAE version 1.0, 2.0 and 3.0) [5]. However this toxicity criteria instrument is the select scoring system used by the majority of investigators to document toxic effects of treatment schedule. This discordance represents interference in the optimal utilization of new therapies and in the evaluation of new drug real benefit and efficacy compared to currently available therapy.
Nowadays radiochemotherapy represents an alternative approach to surgery, in concept of organ preservation, for patients with locally advanced squamous cell carcinomas of the head and neck.
Cisplatin-based chemotherapy and radiotherapy is considered the standard regimen [6], although cisplatin’s acute toxicity can be so severe that most of patients received only two of the recommended three cycles of treatment [7]. To optimize treatment tolerability, a targeted therapy approach was proposed, considering the over expression of Epidermal Growth Factor Receptors (EGFR) in head and neck cancers. The benefit of cetuximab was comparable to advantage reported in cisplatin-based therapy, but considering that a definitive randomized trial comparing radiation therapy plus cisplatin versus radiation therapy plus cetuximab is lacking, it remains only a good indirect comparison [7].
While radiation dermatitis in patients receiving cetuximab concomitantly with radiotherapy is recognized as a common side effects [8-13], oral mucositis is underestimate [9,12-14]. Mucositis is an important consequence of cytotoxic or biologic agents with concurrent radiation in head and neck cancer, and the severity of mucositis may even be higher in EGFR inhibitor therapy compared with cisplatin regimen [13]. The NCI-CTCAE severity grading scale seems to be not so appropriate to measure oral mucositis, because it is based on patient’s ability to eat and drink combined with objective signs of mucositis, but in separate scales (Table 2) [4,14,15]. The severity of pain and the inability to eat correctly were difficult to capture properly from the scale-system and all these unmeasured factors could produce significant bias [16].
| Adverse event | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|
| Mucositis oral (clinical exam) | Erythema of the mucosa | Patchy ulcerations or pseudo membranes | Confluent ulceations or pseudo membranes; bleeding with minor trauma | Tissue necrosis; significant spontaneous bleeding | Death |
| Mucositis oral (functional/ symptomatic) | Asymptomatic or mild symptoms; intervention not indicated | Moderate pain; not interfering with oral intake; modified diet indicated | Severe pain; interfering with oral intake | Life-threatening consequences; urgent intervention indicated | Death |
Table 2: Common Terminology Criteria for Adverse Events (CTCAE) v4.0.
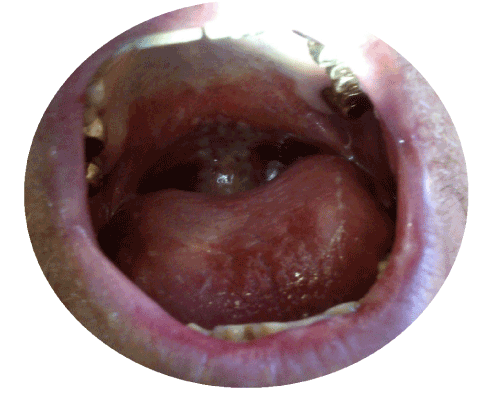
Cetuximab oral mucositis presents with general erythema, less ulcerative but more widespread than classical ulcerative mucositis recorded with radiation and citotoxic agents, such as cisplatin; it is associated with pain, trismus and complete inability to tolerate solids and fluids [16,17]. In our series, further features have emerged: oral mucositis appeared unexpectedly as a severe grade toxicity within about 10-15 days the start of concomitant therapy and it was never associated to specific supra-infections; the evolution and the consequent resolution of clinical discomfort required several days and the treatment with corticosteroids did not represent the solution formula, it was not as effective as in other types of mucositis by other drugs [17-21].
Our study is limited because of the small patients group, but the specific focus of this study was to demonstrate the impact of cetuximab plus radiotherapy on oral mucositis evaluation and not to assess the relation between therapy and response, as various authors have done [9,12-14,18,22]. As such, we chose not to include a correlation between concomitant treatment and control parameters of response.
Limited data exist regarding whether cetuximab increases the rate of oral mucositis in patients with head and neck cancer. Most of the little information derived from randomized-controlled trials that were not designed to compare the mucositis rates among different treatment modalities.
The Bonner’s study [14] claimed concomitant radiotherapy plus cetuximab regimen not exacerbated the common mucositis associated with radiotherapy: mucositis ≥ G3 was recorded in 56% of patients. Lord et al. [9] described only a case of mucositis ≥ G3 in their series of 14 patients. Whereas our data indicate that cetuximab, in combination with local radiation therapy of the head and neck area, emphasized undesirable effects typical of radiation therapy, such as mucositis, radiation dermatitis and dysphagia. Data from day-byday medical practices reflected the true information: the addition of cetuximab improved the risk of mucositis and all patients developed oral mucositis ≥ G3 (Figure 1). An increased risk of oral mucositis also was demonstrated in other trials that used cetuximab in association with radiation therapy. Pryor et al. [12] reported mucositis ≥ G3 in 10 of 13 patients. Walsh et al. [13] identified oral mucositis ≥ G3 in 25 (74%) of their 34 patients. Ang et al. [23] (RTOG trail 0522) also showed an increase of oral mucositis G3/G4 adding Cetuximab to Cisplatin (43% versus 33%).
Therefore an accurate grading of oral adverse events due to EGFR inhibitor is paramount for a correct management strategy, because mucositis can complicate nutritional status, limiting patient’s intake of solids and liquids and can compromise the delivery of scheduled radiation doses. Despite in literature cetuximab figures as a drug with an acceptable tolerability profile [17], oral mucositis, as added toxic effect, must be considered in future head and neck cancer trials and in patients care, because it has a significant impact on regularly treatment and on patient’s quality of life.
Conclusion
There is a paucity of evidence-based data on the evaluation of oral mucositis in the cetuximab-based treatment of locally advanced head and neck squamous cancers. In our experience, Cetuximab induced oral mucositis have the following specific characteristics: time of onset, mode of clinical expression, severity, association with trismus and minimal response to corticosteroid therapy. Studying these peculiarities, we could arrive at the formulation of the physiopathology of Cetuximab induced mucositis and develop most appropriate therapeutic supports.
Considering that the majority of studies do not reveal oral toxicity associated with cetuximab, future clinical trials should focus on specific topics to improve the definition of documented oral toxic effects.
References
- Pico JL, Avila-Garavito A, Naccache P (1998) Mucositis: Its Occurrence, Consequences, and Treatment in the Oncology Setting. Oncologist 3: 446-451
- Naidu MU, Ramana GV, Rani PU, Mohan IK, Suman A, et al. (2004) Chemotherapy-induced and/or radiation therapy-induced oral mucositis--complicating the treatment of cancer. Neoplasia 6: 423-431
- Cancer Therapy Evaluation Program (2010) Common Terminology Criteria for Adverse Events, Version 4.0.
- Edgerly M, Fojo T (2008) Is there room for improvement in adverse event reporting in the era of targeted therapies? J Natl Cancer Inst 100: 240-242.
- Riaz N, Sherman EJ, Fury M, Lee N (2012) Should Cetuximab Replace Cisplatin for Definitive Chemoradiotherapy in Locally Advanced Head and Neck Cancer? J Clin Oncol 31: 287-288.
- Rowan K (2010) Should cetuximab replace cisplatin in head and neck cancer? J Natl Cancer Inst 102: 74-76, 78.
- Budach W, Bölke E, Homey B (2007) Severe cutaneous reaction during radiation therapy with concurrent cetuximab. N Engl J Med 357: 514-515.
- Lord HK, Junor E, Ironside J (2008) Cetuximab is effective, but more toxic than reported in the Bonner trial. Clin Oncol (R Coll Radiol) 20: 96.
- Bölke E, Gerber PA, Lammering G, Peiper M, Müller-Homey A, et al. (2008) Development and management of severe cutaneous side effects in head-and-neck cancer patients during concurrent radiotherapy and cetuximab. Strahlenther Onkol 184: 105-110.
- Giro C, Berger B, Bölke E, Ciernik IF, Duprez F, et al. (2009) High rate of severe radiation dermatitis during radiation therapy with concurrent cetuximab in head and neck cancer: results of a survey in EORTC institutes. Radiother Oncol 90: 166-171.
- Pryor DI, Porceddu SV, Burmeister BH, Guminski A, Thomson DB, et al. (2009) Enhanced toxicity with concurrent cetuximab and radiotherapy in head and neck cancer. Radiother Oncol 90: 172-176.
- Walsh L, Gillham C, Dunne M, Fraser I, Hollywood D, et al. (2011) Toxicity of cetuximab versus cisplatin concurrent with radiotherapy in locally advanced head and neck squamous cell cancer (LAHNSCC). Radiother Oncol 98: 38-41.
- Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, et al. (2006) Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 354: 567-578.
- Bernier J, Russi EG, Homey B, Merlano MC, MesÃa R, et al. (2011) Management of radiation dermatitis in patients receiving cetuximab and radiotherapy for locally advanced squamous cell carcinoma of the head and neck: proposals for a revised grading system and consensus management guidelines. Ann Oncol 22: 2191-2200.
- Lee CC, Ho HC, Hsiao SH, Huang TT, Lin HY, et al. (2012) Infectious complications in head and neck cancer patients treated with cetuximab: propensity score and instrumental variable analysis. PLoS One 7: e50163.
- Watters AL, Epstein JB, Agulnik M (2011) Oral complications of targeted cancer therapies: a narrative literature review. Oral Oncol 47: 441-448.
- Koutcher L, Sherman E, Fury M, Wolden S, Zhang Z, et al. (2011) Concurrent cisplatin and radiation versus cetuximab and radiation for locally advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys 81: 915-922.
- Pignon JP, le Maître A, Maillard E, Bourhis J (2009) MACH-NC Collaborative Group. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 92: 4-14
- Lacouture ME, Maitland ML, Segaert S, Setser A, Baran R, et al. (2010) A proposed EGFR inhibitor dermatologic adverse event-specific grading scale from the MASCC skin toxicity study group. Support Care Cancer 18: 509-522.
- Frampton JE (2011) Spotlight on cetuximab in squamous cell carcinoma of the head and neck. Bio Drugs 25: 129-133.
- Beijer YJ, Koopman M, Terhaard CH, Braunius WW, Van Es RJ, et al. (2012) Outcome and toxicity of radiotherapy combined with chemotherapy or cetuximab for head and neck cancer: our experience in one hundred and twenty-five patients. Clin Otolaryngol 38: 69-74.
- Ang KK, Zhang QE, Rosenthal DI, Nguyen Tan P, Sherman EJ, et al. (2011) A randomized phase III trial (RTOG 0522) of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III-IV head and neck squamous cell carcinomas (HNC). J Clin Oncol 29S: 5500.
Citation: Musio D, De Felice F, Bulzonetti N, Tombolini V (2013) Cetuximab and Oral Mucositis: Is it Different from Oral Mucositis Caused by Other Drugs? Otolaryngology 3:147. DOI: 10.4172/2161-119X.1000147
Copyright: © 2013 Musio D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 14904
- [From(publication date): 11-2013 - Apr 07, 2025]
- Breakdown by view type
- HTML page views: 10273
- PDF downloads: 4631