Research Article Open Access
Silver-N-Carboxymethyl Chitosan Nanocomposites: Synthesis and its Antibacterial Activities
Nguyen Tien An1*, Nguyen Thi Dong1, Pham Thi Bich Hanh1, Tran Thi Y Nhi1, Duong Anh Vu1, Do Thi Nguyet Que2 and Do Truong Thien1
1Institute of Chemistry – VAST, 18- Hoang Quoc Viet road – Hanoi –Vietnam
2Hanoi Pharmaceutical University, Hanoi, Vietnam
- *Corresponding Author:
- Nguyen Tien An
Natural Polymer Laboratory
Institute of Chemistry – VAST
18- Hoang Quoc Viet road, Hanoi, Vietnam
Email: nguyentienanvhh@gmail.com
Received Date: September 28, 2010; Accepted Date: November 12, 2010; Published Date: November 15, 2010
Citation: An NT, Dong NT, Hanh PTB, Nhi TTY, Vu DA, et al. (2010) Silver-N-Carboxymethyl Chitosan Nanocomposites: Synthesis and its Antibacterial Activities. J Bioterr Biodef 1:102. doi: 10.4172/2157-2526.1000102
Copyright: © 2010 An NT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Bioterrorism & Biodefense
Abstract
In this work, silver-N-carboxymethyl chitosan nanocomposites (Ag-N-CMC) were synthesized in the homogeneous state via the reduction of Ag+ (using [Ag(NH3)2]OH instead of AgNO3) by NaBH4 in the presence of water-soluble N-carboxymethyl chitosan as a stabilizer. The resulting Ag-N-CMG was characterized by FTIR, TEM and UV-vis spectra. The results showed that the average particle size of silver nanoparticles was little affected by the concentration of Ag+ added and was between 2 and 10nm. The characteristic surface plasmon resonance band of silver nanoparticles centered at about 398 - 410nm. In vitro antibacterial activities of Ag-N-CMC nanocomposites were evaluated against both gram-negative bacteria: Escherichia coli ATCC 25922 (Ec) and Pseudomonas aeruginosa VM201 (Pseu) and gram-positive bacteria: Staphylococcus aureus ATCC 1128 (Sta) and Bacillus cereus ATCC 9946 (Bc). The results shown that the Ag-N-CMC nanocomposites could inhibit the growth and multiplication of the tested bacteria.
Keywords
N-carboxymethyl chitosan; Antibacterial; Nanoparticles; Nanocomposites
Introduction
The preparation of metal nanoparticles is a major research area in nanoscale science and engineering given their unusual chemical and physical properties, such as catalytic activity, novel electronic, optic and magnetic properties and their potential application in biotechnology. Numerous studies have been performed on the preparation of new organic/inorganic hybrid materials and nanocomposites such as metal-polymer nanoparticles. Silver nanoparticles have also attracted much attention due to their diminutive size and novel material properties. With their nanometer scale size, which is responsible for different properties concerning the bulk material renders them suitable for applications. Therefore, many approaches have been used to prepare silver nanoparticles for a rapidly growing list of catalysis, electronic, non-linear optics and biomaterial applications [1-5]. In the preparation of silver nanoparticles, the stabilizer plays an important role in retaining the stabilization of nano system.
For this reason, in this research, the N-carboxymethyl chitosan, a chitosan’s derivative, was used as a stabilizer for preparing Ag-N-CMC nanocomposites. The resulting Ag-N-CMCs were characterized by FTIR, UV-vis spectra and TEM photographs; and evaluated the antibacterial activity of it with some of gram-negative and grampositive bacteria in vitro.
Materials and Methods
Materials
N-carboxymethyl chitosan was synthesized according to the method mentioned in reference [6,7]. Silver nitrate, ammonium hydroxide, sodium borohydride were purchased from Merck Co., (Germany). All other chemicals and reagents used in experiment were of analytical grade.
Bacterial strains both gram positive bacteria: Staphylococcus aureus ATCC 1128 (Sta); Bacillus cereus ATCC 9946 (Bc); and gram negative bacteria: Escherichia coli ATCC 25922 (Ec); Pseudomonas aeruginosa VM201 (Pseu) which were multi drug resistant strains provided by Hanoi Pharmaceutical University-Vietnam cultures. These bacteria were maintained at 4°C on nutrient agar slants.
Preparation and characterization of N-CMC- silver nanocomposites
A solution of N-CMC (1g/100ml) in distilled water was stirred until a clear solution was obtained. [Ag(NH3)2]OH aqueous solution was prepared by reaction of AgNO3 solution with an excess amount of NH3 solution. The Ag-N-CMC nanocomposites were synthesized as follow: 0.1ml of 1; 10 and 20mM [Ag(NH3)2] OH aqueous solution was mixed with 50ml of 1.0g/100ml N-CMC, the mixture was stirred for 30 min, then the aqueous solution of NaBH4 (0.5ml, 0.2 mM) was dropped to the mixture with stirring, the reaction was carried out at room temperature for 90 min. The Ag-N-CMC nanocomposites were collected by precipitation and washing many times with 90% ethanol solution. The resulting nanocomposites were kept at room temperature for characterization.
FTIR spectra of the N-CMC derivative and Ag-N-CMC nanocomposites were recorded on the FTIR-Impact 410 spectrometer in the range between 4000 and 400cm-1.
Transmission electron microscopy (TEM) was carried out with a JEOL-2000EX operating at 80kV.
UV-vis absorbance spectra were collected using a GBC Instrument-2855 spectrophotometer.
Antibacterial assay
The antibacterial activities of Ag-N-CMC nanocomposites were evaluated using the disc diffusion according to the method of Shirley et al. [8]. Briefly, pure cultures were subcultued in Muller Hinton broth for 24 h at 37°C. Wells of 5 mm diameter were made on Muller Hinton agar plates using gel puncture. Each strain was swabbed uniformly into the individual plates using sterile cotton swabs. Then 20 µlts (0.002mg) of the sample of nanoparticle solution was poured onto each of the wells at the centre in all the plates. After incubation at 35°C for 24 h the different levels of zone of inhibition were measured using a centimeter scale. The comparative stability of discs containing penicillin was made.
Results and Discussion
Synthesis of Ag-N-CMC nanocomposites
In recent years, the preparation of silver nanocomposites have been made with increasing frequency. In those processes, the stabilizers such as chitosan and its derivatives play an important role in retaining the stabilization of nano system. Chitosan could be used as both reducing and stabilizing agents in the synthesis of silver nanoparticles due to its oxygen-rich structures in hydroxyl and ether groups, which lead to a tightly bind with metal clusters and nanoparticles via electrostatic interactions. However, the poor solubility of chitosan in neutral and alkaline solution resulted in the only acidic reducing condition for silver cations. As a carboxymethylated derivative of chitosan, N-CMC has good solubility in alkaline aqueous solution because of the introduction of carboxymethyl groups. However, according to Twu et al, N-carboxymethyl chitosan was not suitable for preparation Ag nanoparticles because of forming large insoluble silver chelate after addition of AgNO3 solution [2]. Hence, homogeneous processes were discarded. But this could be overcomed by using [Ag(NH3)2] OH instead of AgNO3. Ag nanoparticles were formed by reduction of Ag+ with HCHO as following:
2Ag+ + 2NaBH4 → 2Ag + B2H6 + H2 + 2Na+.
Characterization of Ag-N-CMC nanocomposites
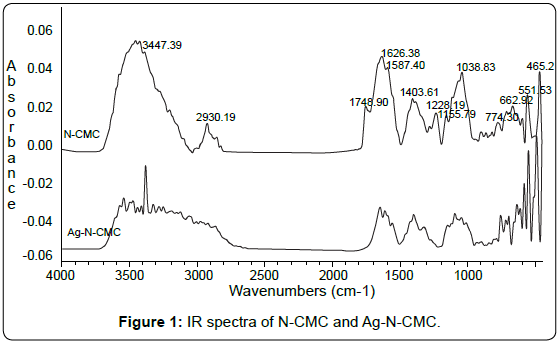
FTIR analysis: To identify the possible interaction sites between N-CMC and silver nanoparticles, the FTIR spectra for pure N-CMC and Ag-N-CMC nanocomposite were measured and shown in Figure 1.
The characteristic absorption peaks of N-CMC derivative were observed at 3000÷4000cm-1 (-OH); 1748cm-1 (typical for C=O of – COOH group); 2930cm-1 (νasCH2); 1626cm-1 (C=O of –COONa and NHCOCH3); 1587cm-1 (-NH); 1403cm-1; 1155cm-1 (δCH2); 1038cm-1 (CO). As shown in Figure 1, comparing with the spectrum of N-CMC, the significant decrease of transmittance in the broad band at 3300-3500cm-1 indicates that the N-H vibration was affected due to the silver attachment. The N-H bending vibration band at about 1587cm-1 was shifted to 1580cm-1 also suggests the attachment of silver to nitrogen atoms, which reduced the vibration intensity of the N-H bond due to the increasing molecule weight because of silver binding. In other words, nitrogen atoms are bound to Ag nanoparticles during reduction of Ag+ by NaBH4. It was also seen in the Figure 1 that the intensity of peak typical for C=O vibration was decreased dramatically. This could be due to the C=O groups have been reduced by NaBH4 for forming OH groups.
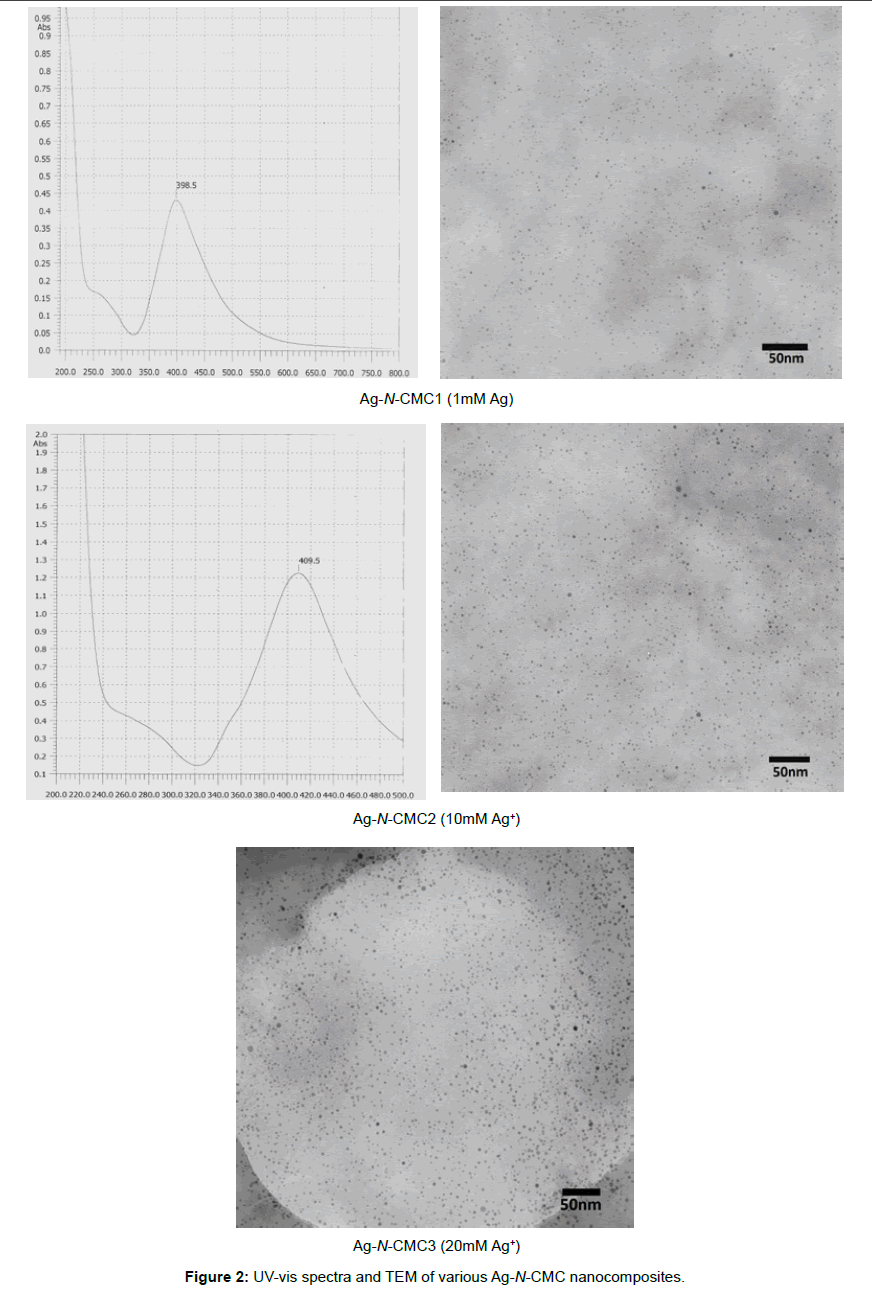
UV-vis spectra and TEM of Ag-N-CMC nanocomposites: Silver nanoparticles absorb radiation in the visible region of the electromagnetic spectrum (ca. 380-450nm) due to the excitation of surface plasmon vibrations and this is responsible for the striking yellow-brown color of silver nanoparticles in various media [1]. The UV-vis absorption spectrum of the silver nanoparticles solution is shown in the Figure 2 Silver nanoparticles absorb radiation in the visible region of the electromagnetic spectrum (ca. 380-450nm) due to the excitation of surface plasmon vibrations and this is responsible for the striking yellow-brown color of silver nanoparticles in various media [1]. The UV-vis absorption spectrum of the silver nanoparticles solution is shown in the Figure 2
The spectra exhibited absorption bands at about 398÷410nm, which was a typical plasmon band, suggesting the formation of silver nanoparticles. It could be also seen that when the concentration of Ag+ increased, the absorbance wavelength slightly shifted to longer wave. The position and shape of the plasmon absorption depended on the particles size, shape and the dielectric constant of the surrounding medium. In this case, the shifting of surface plasmon absorption maximum from 398nm to 410nm was due to the difference in particles size. This was supported by TEM observations. As shown in Figure 2, virtually highly dispersed silver nanoparticles were obtained at 1mM; 10 mM and 20 mM Ag+. The average particle size of silver nanoparticles obtained from reducing silver ion by NaBH4 agent was about 2÷10nm and little affected by the concentration of Ag+ added. The results were also shown that the N-carboxymethyl chitosan derivative could be a good stabilizer for synthesis of silver nanoparticles.
Antibacterial activity of Ag-N-CMC nanocomposites
The antibacterial activity of penicillin and Ag-N-CMCs with the bacterial strains were shown in Table 1. It was found that these samples shown effectively antibacterial activities against bacterial trains, which were used in the test, although differences existed among them, especially, the antibacterial activity of Ag-N-CMCs to Pseudomonas strain has more strong than that of penicillin. The results also shown that the antibacterial activity of Ag-N-CMCs decreased with increasing the size particles of silver nanoparticles. The mechanism of the bactericidal effect of Ag-CMGs against the bacteria was not very well-known. Silver nanoparticles might attach to the surface of the cell membrane and disturbed its power function such as permeability and respiration. It was reasonable to state that the binding of the particles to the bacteria depended on the surface area available for interaction. Smaller particles having the larger surface area available for interaction would give more bactericidal effect than the larger particles.
| Samples | Diameter of inhibition (mm) | |||
| Bc | Sta | Ec | Pseu | |
| Ag-N-CMC1 | 13.78 | 13.32 | 12.55 | 13.57 |
| Ag-N-CMC2 | 13.21 | 11.18 | 12.04 | 12.87 |
| Ag-N-CMC3 | 12.72 | 10.36 | 11.24 | 12.03 |
| Penicillin | 18.16 | 22.35 | 17.71 | 11.21 |
Table 1: Zone of inhibition of Ag-N-CMC nanocomposites and penicillin.
Conclusions
Silver-N-carboxymethyl chitosan nanocomposites have been synthesized in the homogeneous state via the reduction of silver ions by NaBH4 agent in the presence of N-carboxymethyl chitosan as a stabilizer. Water soluble N-carboxymethyl chitosan derivative shown as a good stabilizer for synthesis of silver particles. In this case, the average particle size of silver nanoparticles was little affected with the concentration of Ag+ added and was ranged between 2 and 10nm. The UV-vis absorption spectra recorded from the resulting solutions showed the characteristic surface plasmon resonance band of Ag nanoparticles centered at about 398-410nm. The investigation antibacterial activity of Ag-N-CMC nanocomposites against some bacterial strains of both gram positive and gram negative strains was tested in vitro shown that the Ag-N-CMGs could inhibit the growth and multiplication of the tested bacteria, especially to Pseu train.
References
- Huang H, Yang X (2004) Synthesis of polysaccharide-stabilized gold and silver nanoparticles: a green method. Carbohydr Res 339: 2627-2631.
- Twu Y, Yu-Wan C, Chao-Ming S (2008) Preparation of silver nanoparticles using chitosan suspensions. Adv Powder Technol 185: 251-257.
- Dongwei W, Weiping Q (2008) Facile synthesis of Ag and Au nanoparticles utilizing chitosan as a mediator agent. Colloids Surf B Biointerfaces 62: 136- 142.
- Masatoshi S, Minoru M, Hitoshi S, Hiroyuki S, Yoshihiro S (1998) Preparation and characterization of water-soluble chitin and chitosan derivatives. Carbohydr Polym 36: 49-59.
- Dung PL, Thien DT, Dong NT, Nhi TTY, An NT (2005) Water-soluble N-substituted chitosan derivatives. Asean Journal on Science and Technology for Development 22: 261-270.
- An NT, Dung PL, Thien DT, Dong NT, Nhi TTY (2008) An improved method for synthesizing N,N-dicarboxymethylchitosan. Carbohydr Polym 73: 261–264.
- Nguyen TA, Do Truong T, Nguyen TD, Pham LD (2009). Water-soluble N-carboxymethylchitosan derivatives: Preparation, characteristics and its application. Carbohydrate Polymers 75: 489–497.
- Shirley A, Dayanand B. Sreedhar, Syed G Dastasger (2010) Antimicrobial activity of silver nanoparticles synthesized from novel Streptomyces species. Digest Journal of Nanomaterials and Biostructures 5: 447-451.
Relevant Topics
- Anthrax Bioterrorism
- Bio surveilliance
- Biodefense
- Biohazards
- Biological Preparedness
- Biological Warfare
- Biological weapons
- Biorisk
- Bioterrorism
- Bioterrorism Agents
- Biothreat Agents
- Disease surveillance
- Emerging infectious disease
- Epidemiology of Breast Cancer
- Information Security
- Mass Prophylaxis
- Nuclear Terrorism
- Probabilistic risk assessment
- United States biological defense program
- Vaccines
Recommended Journals
Article Tools
Article Usage
- Total views: 16288
- [From(publication date):
October-2010 - Dec 20, 2025] - Breakdown by view type
- HTML page views : 11434
- PDF downloads : 4854