Research Article Open Access
XRD Elaborates the Metamorphosis in Graft Copolymers
Ashish Chauhan* and Balbir Kaith
Department of Chemistry, Dr. B.R.A. National Institute of Technology, Jalandhar, India
- *Corresponding Author:
- Ashish Chauhan
Jr. Scientist, Department of Chemistry
Dr. B.R.A. National Institute of Technology
Jalandhar - 144011, Punjab, India
E-mail: aashishchauhan26@gmail.com
Received date: July 27, 2012; Accepted date: September 27, 2012; Published date: October 03, 2012
Citation: Chauhan A, Kaith B (2012) XRD Elaborates the Metamorphosis in Graft Copolymers. J Anal Bioanal Tech 3:148. doi: 10.4172/2155-9872.1000148
Copyright: © 2012 Chauhan A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Sereni is present in abundance through-out the world. Its stem fiber has high tensile strength and low density that promotes it to serve as a backbone for graft-copolymerization. This cellulosic fiber was graft copolymerized by binary vinyl monomeric mixture using ceric ammonium nitrate-nitric acid as initiator system. The graft co-polymers were characterized by SEM, FTIR and XRD. The comparative evaluation of graft copolymers by x-ray diffraction studies revealed the change in morphology and nature of the fiber. The results obtained by XRD were supported by the physcio-chemico-thermal transition in the properties and behavior of the fiber. These modified graft copolymer could be used in numerous scientific and bio-medical applications.
Keywords
Graft copolymerization; Methyl acrylate; Butyl acrylate; Cellulose
Introduction
Natural fibers are assets to the man on the earth. They have been useful for the basic needs of the mankind since long. As they are renewable resource so they can also be utilized for various secondary needs. Many attempts have been made to explore their fullest potential yet a lot remains undiscovered. The natural fibers suffer from various draw-backs such as low weather-stability, fast-decaying and low chemical resistance. Amongst various methods prevalent to improve these graft copolymerization of vinyl monomers onto natural backbones has been an interesting means to reduce the ageing in textiles, enhancing the mechanical properties and modifying the texture. Graft copolymerization of vinyl monomers onto cellulose and other macromolecules has been extensively studied with various monomers and methods [1-5]. Numerous scientists have explored the potential of this chemical technique on different natural fibers and used latest advanced means of characterization and evaluated the properties for their variable applications [5-15].
It redefines the properties of polymer back-bone by incorporating the desired features without drastically affecting the basic traits of the substrate. X-ray diffraction is an important technique to characterize the polymers and screen the differences brought about by graft copolymerization. Since, the plant does not possess any significant biomedical use but the low density, non-abrasive, high specific mechanical strength in the stem fiber motivated to use it as backbone for graft copolymerization to explore the virgin Sereni (Hibiscus sabdariffa) fiber by using the rare binary vinyl monomeric mixture of methyl acrylate (MA) as principal monomer with acrylic acid (AA), butyl acrylate (BA), acrylamide (Aam), acrylonitrile (AN), vinyl acetate (VA), 4-vinyl pyridine (4-VP) as secondary monomers that has never been studied before. Many attempts have been made before to utilize the fiber existing as waste bio-mass [16-24], but this paper is an attempt to present a comparative evaluation of change in the graft yield of the fiber after copolymerization and its depiction and correlation with X-ray diffraction studies, that still remains unexplored. These results were further supported by the inference obtained from the characterization by FTIR, SEM and physcio-chemico-thermal evaluation of the graft copolymers.
Experimental
Materials
H. sabdariffa fiber was obtained from the Department of Agronomy, Chaudhary Sarwan Kumar Himachal Krishi Vishwavidyalaya, Palampur (H.P.) India. Monomers (Merck) and Ceric ammonium nitrate (s. d. fine-Chem, Pvt. Ltd, Mumbai, India) were used as received. H. sabdariffa fiber was purified through soxhlet extraction in acetone for 72 hours.
Graft copolymerization
Graft copolymerization of the monomer onto H. sabdariffa was carried-out for optimization of different reaction conditions like reaction time, reaction temperature, monomer concentration, concentration of initiator system and pH in order to obtain maximum graft yield. The fiber (0.5 g) was activated by swelling in 100 ml of the distilled water for 24 hrs. Mixture of Ceric Ammonium Nitrate (CAN) and Conc. HNO3 mixture was slowly added to the reaction medium with continuous stirring followed by the drop by drop addition of a definite ratio of binary vinyl monomeric mixture. The reaction was carried-out at a definite temperature for a particular time interval. On completion of the reaction, poly(MA), poly(MA-co-BA), poly(MAco- AA), poly(MA-co-AN), poly(MA-co-Aam), and poly(4-VP) were removed on extraction with acetone, di-methyl formamide, alcohol, chloroform, methanol and water. The graft co-polymer was dried at 50°C, till a constant weight was obtained. The percent grafting (Pg) was calculated as per the reported method [1-3,25]:
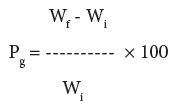 (1)
(1)
where, Wf = final weight of the fiber, Wi = initial weight of the fiber.
Physico-chemical evaluation
Moisture absorption study: Moisture absorbance studies were carried-out at various relative humidity levels. Moisture absorbance percentage was found by placing a known weight (Wi) of dry grafted and ungrafted samples in a humidity chamber for about twelve hours and then the final weight (Wf) of the samples exposed to different relative humidities ranging from 30-90% were taken at ambient temperature. The moisture absorbance was calculated by the Eq. 2 [1-3,25]:
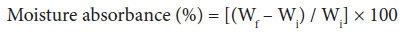 (2)
(2)
Acid and base resistance: Acid and base resistance studies were carried-out as shown in Eq. 3. A known weight (Wi) of dry grafted and ungrafted samples in fixed volume of 1N HCl and 1N NaOH was kept and the final weights (Wf) of the samples was observed after 72 hours, under ambient conditions [1-3,25].
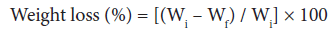 (3)
(3)
Swelling behavior in different solvents: 250 mg of grafted and raw sample was immersed in a definite volume (100 ml) of water, methanol, n-butanol and dimethyl-formamide under ambient conditions for a period of 24 hours. Samples were removed from the solvent and excess solvent was removed with filter paper. Final weight of the sample was taken and the percentage of swelling was calculated as follows [1-3,25]:
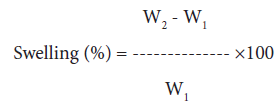 (4)
(4)
where, W1 and W2 are the initial and final weights of samples, respectively.
Dye uptake behavior: 0.1% Gentian violet solution was prepared in distilled water. 10% NaCl solution and a few drops of acetic acid were added to this solution. Dye uptake of the raw fiber and its graft copolymers were carried-out by immersing the known weight of the sample in 100 ml of Gentian violet dye. Optical densities of the test solutions were observed using Digital Photo Colorimeter for seven consecutive hours and the concentrations of test solution were calculated as [1-3,25]:
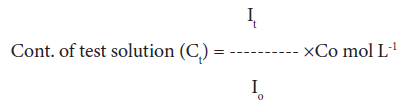 (5)
(5)
where, Io, It, and Co are optical density of standard solution, optical density of test solution and concentration of standard solution, respectively.
Characterization
IR spectra of the H. sabdariffa and its graft copolymers were recorded with Perkin Elmer Fourier Transform Infrared (FTIR) spectrophotometer using KBr pellets (Sigma Aldrich). Scanning Electron Micrographs (SEM) of H. sabdariffa and its graft copolymers were obtained by using Electron Microscopy Machine (LEO 435-25-20). X-ray diffraction studies were performed on X-ray diffractometer (Bruker D8 Advance) under ambient conditions using Cu Kα (1.5418°A) radiation, Ni-filter and scintillation counter as detector at 40 KV and 40 mA on rotation between 13° to 25° at 2θ scale at 1 sec. step size and increment of 0.01° with 0.5° or 1.0 mm of divergent and anti-scattering slit. Corundum was the reference sample that verifies and calibrates the instrument.
The continuous scans were taken and different d-spacings (Å) and relative intensities (I) were obtained. Crystallinity index (C.I.) which measures the orientation of the cellulose crystals with respect to fiber axis was determined by using the wide angle X-ray diffraction counts at 2θ-scale close to 22.68° and 15°. The counter reading at the peak intensity at 22.68° represent the crystalline material and the peak intensity at 15° corresponds to the amorphous material in cellulose. Percentage Crystallinity (%Cr) and Crystallinity index (C.I.) were calculated as follows [25,26]:
% Cr = [I22.68/ (I22.68 + I15)] × 100 EQ(6)
C.I. = [(I22.68 – I15) / I22.68] EQ(7)
where, I22.68 and I15 are the crystalline and amorphous intensities at 2θ-scale close to 22.68° and 15°, respectively.
Results and Discussion
Mechanism
Ceric ion forms complex with the cellulose through C-2 and C-3 hydroxyl groups of the anhydro glucose unit. Transfer of the electron from the cellulose molecule to Ce (IV) would follow, leading to its reduction to Ce (III), breakage of –OH bonds at C-2 and C-3 and the formation of the free radical sites where the monomeric chains get grafted. Graft yield and homo-polymer formation have been found to be the functions of both the monomer and initiator concentration [25].
During the graft copolymerization of MA onto H. sabdariffa fiber, the different optimized reaction parameters to obtain the maximum graft yield (60.24%) were: temperature (°C), 35; time (minute), 120; CAN (mol L-1), 1.49×10-4; HNO3 (mol L-1), 3.36×10-3; MA (mol L-1), 2.21×10-3 and pH, 7.0. Since, it is hard to justify the results in Table 1, merely on the basis of concentration of binary vinyl mixtures, therefore, it is important to consider the reactivity, nature and behavior of monomer species. It is clear that the reactivity of the acrylate monomers in reference to styrene radicals is normally higher. Since, the carboalkoxy group is highly electronegative; therefore, it causes the acrylate double bond to be positive in character. Methyl acrylate radical has hyper conjugative resonance with methyl hydrogen as well as with the carboxy group. The polar character of the acrylates results in the penultimate group effects in both propagation and the termination reactions. Methyl acrylate as a principal monomer has relative high reactivity, suitable chemical properties (Kt, Kp /Kt, CM) needed for grafting onto H. sabdariffa backbone that results in Pg: 60.24.
| Sample | Binary mixture (×10-3mole L-1) | Mean Pg | ± SD | ± SE |
|---|---|---|---|---|
| Hs-g-poly (MA+AN) |
2.21+3.03 | 68.08 | ± 7.18 | ± 4.15 |
| 2.21+3.77 | 100.12 | ± 7.13 | ± 4.12 | |
| 2.21+4.56 | 100.64* | ± 4.54 | ± 2.62 | |
| 2.21+5.28 | 57.06 | ± 6.26 | ± 3.61 | |
| 2.21+6.07 | 52.77 | ± 3.55 | ± 2.05 | |
| Hs-g-poly (MA+AA) |
2.21+0.72 | 18.00 | ± 4.47 | ± 2.58 |
| 2.21+1.45 | 74.14 | ± 2.68 | ± 1.54 | |
| 2.21+2.17 | 42.35 | ± 3.53 | ± 2.04 | |
| 2.21+2.91 | 10.28 | ± 4.40 | ± 2.55 | |
| 2.21+3.62 | 10.04 | ± 3.45 | ± 2.00 | |
| Hs-g-poly (MA+AAm) |
2.21+0.70 | 20.22 | ± 1.78 | ± 1.02 |
| 2.21+1.05 | 23.75 | ± 4.42 | ± 2.55 | |
| 2.21+1.40 | 15.54 | ± 3.44 | ± 1.98 | |
| 2.21+1.75 | 14.00 | ± 3.57 | ± 2.06 | |
| 2.21+2.10 | 12.38 | ± 1.96 | ± 1.13 | |
| Hs-g-poly (MA+VA) |
2.21+2.16 | 113.40 | ± 5.36 | ± 3.09 |
| 2.21+2.70 | 120.00 | ± 7.15 | ± 4.13 | |
| 2.21+3.24 | 138.52* | ± 5.59 | ± 3.23 | |
| 2.21+3.78 | 96.16 | ± 5.36 | ± 3.09 | |
| 2.21+4.32 | 61.04 | ± 1.78 | ± 1.02 | |
| Hs-g-poly (MA+BA) |
2.21+0.35 | 29.80 | ± 3.57 | ± 2.06 |
| 2.21+0.70 | 47.14* | ± 2.68 | ± 1.54 | |
| 2.21+1.05 | 43.62 | ± 4.44 | ± 2.56 | |
| 2.21+1.40 | 29.08 | ± 3.45 | ± 1.99 | |
| 2.21+1.75 | 28.02 | ± 5.38 | ± 3.10 | |
| 2.21+0.92 | 11.50 | ± 0.98 | ± 0.50 | |
| Hs-g-poly (MA+4VP) |
2.21+1.38 | 11.30 | ± 1.87 | ± 1.08 |
| 2.21+1.85 | 7.70 | ± 1.70 | ± 0.98 | |
| 2.21+2.30 | 5.00 | ± 1.71 | ± 0.98 |
where,* refers to the effective Pg.
Table 1: Effect of the binary mixtures on Pg using MA as a principal monomer.
Table 1, depicts the effect of binary vinyl monomeric mixtures on Pg. Some binary vinyl monomeric mixtures yielded an effective graft yield whereas the other did not. The monomer reactivity ratio (r1:r2) between MA and VA is 0.8:0.23 therefore, it is quite evident that Pg (138.8) should be higher in its binary mixture, which is further supported by high value of CM (1.45×104, 2.4×104, 10.7×104), high Kp/ Kt value [37.2×106 (25°C), 13.4×106 (35°C), 22.6×106 (50°C)]. In case of MA+AN binary mixture, the monomeric reactivity ratio (r1:r2) is (1.25 ± 0.15: 0.86 ± 0.05 at 30°C) and the value of Kp is (28000×10-6 l/mol.s (25°C), 14500×10-6 l/mol.s (25°C), 32500×10-6 l/mol.s (30°C)] which again supports the high graft yield (Pg: 100.64). However, in case of MA+AA binary mixture, r1: r2 ratio (0.98 ± 0.02: 0.42 ± 0.02 at 45°C) is quite satisfactory but due to more association of AA with water, low free radical transfer to the backbone (due to low CM) results in low Pg (74.14). A low grafting (Pg: 47.14) has been found in case of MA+BA binary mixture which is due to the fact that since BA and MA both have relatively less affinity towards reaction medium and ultimately results in low transfer of the active chain propagating species towards the active backbone. In case of acrylamide, a low graft yield (23.75%) was observed as in this case Kp value is higher but CM value is low which results in more homo-polymerization.
However, many other factors also determine the graft yield like the type of fiber, swelling, number of active sites, the nature and amount of the solvent and temperature of polymerization strongly influence the reactivity ratios. In absence of monomer rich phase, the diluents will compete with the monomers for adsorption sites. The amount of adsorption will depend upon the total amount of surface area present and this in turn, is dependent upon the rate of stirring. Physical factors like mixing efficiency determines the melt temperature, the pressure, the rheological properties, solubility of the initiator and the monomer. Elevated temperature favors the degradation, reduces the initiator half life, modifies the rate or specificity of the reaction, influences the solubility and rheological parameters may affect the graft yield [25,27-30].
Characterization
Fourier Transformer Infrared Spectroscopy (FTIR): H. sabdariffa fiber showed a broad peak at 3422 cm-1 (bound -OH groups) and at 2924.8, 1454 and 1055 cm-1 (-CH2, C-C and C-O stretching, respectively). In case of methyl acrylate as a principal monomer, additional peaks due to MA (carbonyl stretch) appeared in all the cases and other incorporated functional groups of secondary monomers were witnessed at 1635.0 cm-1 (vinyl group) in Hs-g-poly(MA-co-VA); 2236.1 cm-1 (-CN group) in Hs-g-poly(MA-co-AN); 1639.4 cm-1 (vinyl group) in Hs-g-poly(MA-co-4VP); 3369.7 cm-1 (N-H stretch) of amide in Hs-g-poly(MA-co-AAm); 1649.7 cm-1 (>C=O of acid) in Hs-g-poly(MA-co-AA) and 1741.5 cm-1 (>C=O stretch) in Hs-g-poly(MA-co-BA).
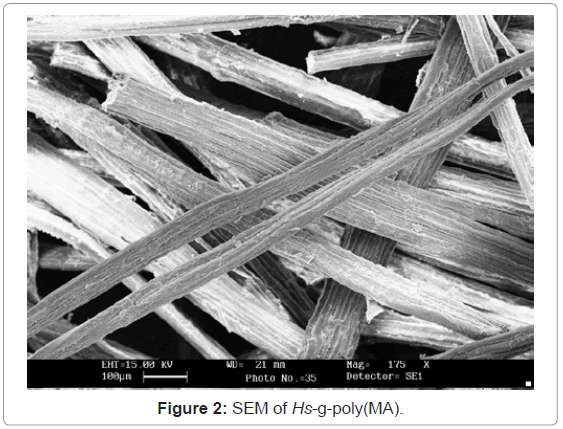
Scanning Electron Microscopy (SEM): In SEM, the cellulose was gold plated to have an impact. It is quite evident from the figures 1-3 that there has been a sufficient deposition of polyvinyl monomers onto fiber. Comparison of the scanning electron micrographs of the raw H. sabdariffa fiber with the highest grafted graft copolymer reveals the distinction between the ungrafted and grafted samples on the basis of the accumulation of poly vinyl monomer onto the fiber, depending upon the Pg.
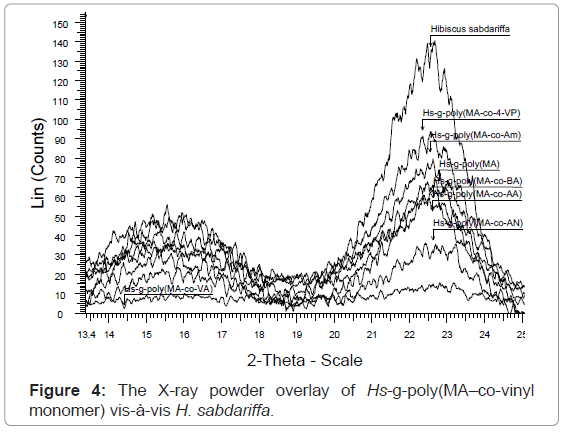
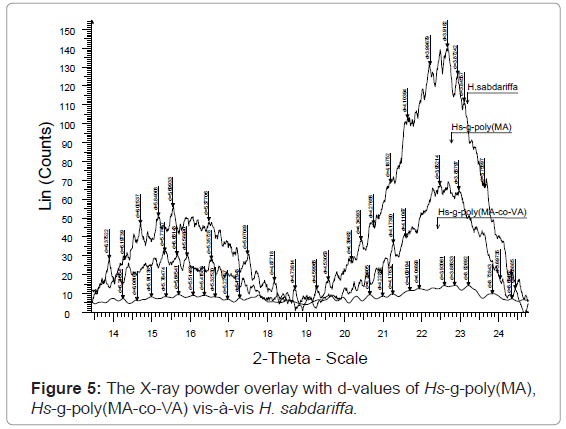
X-ray diffraction studies of graft copolymers (XRD): Hibiscus sabdariffa is rich in cellulose content (73.9%). Cellulosic fiber possesses both crystalline and amorphous regions. The X-ray pattern of the crystalline polymer shows sharp peaks associated with the region of three dimensional order and the diffused features are characteristics of the molecularly disordered substances coexisting within itself. Lower crystallinity means higher amorphous content that may be more accessible to chemicals (vinyl monomers) and water. Crystallinity is correlated to the strength of the fiber. On grafting, the crystal lattice of the polymer is disrupted but the strength of the monomers incorporated reinforces the strength in the structure. However, if crystallinity is not affected on grafting then continuous increase in strength can be obtained with increase in Pg.
The XRD result obtained in figures 4, 5 and Table 2, clearly shows that the percentage crystallinity and crystallinity index was found to decrease with increase in percentage grafting. The X-ray spectrum of raw fiber is more complex than that of graft copolymers. In case of raw Hibiscus sabdariffa fiber the % Cr (77.20) and C.I (0.70) are higher and the incorporation of the monomer chains to the back-bone has impaired the crystallinity. There was significant conversion of the sharp peaks in crystalline raw form to the hallo pattern in the highest grafted Hs-g-poly (MA-co-VA) due to the accumulation of the amorphous content. For Hs-g-poly (MA-co-VA) percentage crystallinity (60.0) and crystallinity index (0.33) decreased significantly as compared to the raw fiber. Therefore, on grafting there was reduction in its stiffness and hardness. Crystallinity index gives a quantitative measure of the orientation of the cellulose crystals in the fibers. A lower crystalline index in case of graft co-polymers means poor order of arrangement of cellulose crystals in the fiber that is due to dis-orientation of the cellulose crystalline lattice to the fiber axis during grafting. The individual diffraction pattern of the raw H. sabdariffa fiber, Hs-g-poly (MA) and highest grafted fiber Hs-g-poly(MA-co-VA) depicts the d-values and further elaborates the differences between the two on the basis of the peak type and peak intensity. However, the diffractogram of the unmodified Hibiscus sabdariffa fiber and the treated samples confirmed that cellulose I form remained unchanged after graft copolymerization under the optimized reaction conditions. This change in morphology due to accumulation of amorphous content will alter the nature and properties of the fiber [25,26].
| Sample | Pg | 2θ Scale | %Cr | C.I. | |
|---|---|---|---|---|---|
| I15 | I22.68 | ||||
| H. sabdariffa Hs-g-poly(MA) | - 60.24 |
40 31 |
136 68 |
77.20 68.68 |
0.70 0.54 |
| Hs-g-poly(MA-co-4VP) | 11.50 | 56 | 93 | 65.03 | 0.39 |
| Hs-g-poly(MA-co-Am) | 23.75 | 43 | 79 | 64.75 | 0.45 |
| Hs-g-poly(MA-co-BA) | 47.14 | 35 | 62 | 63.91 | 0.43 |
| Hs-g-poly(MA-co-AA) | 74.14 | 32 | 55 | 63.21 | 0.41 |
| Hs-g-poly(MA-co-AN) | 100.64 | 16 | 32 | 66.66 | 0.50 |
| Hs-g-poly(MA-co-VA) | 138.52 | 08 | 12 | 60.00 | 0.33 |
Table 2: The % Cr and C. I. of the H. sabdariffa fiber and its graft copolymers.
XRD can easily elaborate the difference in nature, morphology and properties of the raw and the grafted fiber by overlaying the scan in diffractogram. The result obtained in Table 1 ,2 and figures 1-5 are further supported by the results obtained in tables 3-5. It is because of the accumulation of the amorphous content with increase in Pg.
| Sample | Pg | % Chemical resistance % wt. loss after 72 hours |
% Moisture absorbance at different RH after 12 hours |
||||
|---|---|---|---|---|---|---|---|
| Hs-g-poly- | In 1N HCl | In 1N NaOH | 30-35% | 50-55% | 60-65% | 85-90% | |
| H. sabdariffa | - | 55.0 | 43.0 | 0.5 | 0.8 | 1.8 | 2.5 |
| (MA) | 60.24 | 39.0 | 27.0 | - | 0.1 | 1.4 | 1.8 |
| (MA-co-VA) | 138.52 | - | - | - | - | 0.2 | 0.4 |
Table 3: Chemical resistance and moisture absorbance behavior of graft copolymers vis-à-vis back bone.
| Sample | Pg | % Swelling | |||
|---|---|---|---|---|---|
| Water | MeOH | BuOH | DMF | ||
| H. sabdariffa | - | 59.00 | 46.00 | 38.00 | 40.00 |
| Hs-g-poly(MA) | 60.24 | 29.00 | 26.00 | 44.00 | 60.00 |
| Hs-g-poly(MA-co-VA) | 138.52 | 12.00 | 08.00 | 71.00 | 82.00 |
Table 4: Effect of solvents on swelling behavior of graft copolymers vis-à-vis backbone.
| Sample Hs-g-poly- |
Pg | Dye concentration of the test solution at different time intervals (×10-4 mol L-1) | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 h | 2 h | 3 h | 4 h | 5 h | 6 h | 7 h | ||
| H. sabdariffa | - | 4.96 | 4.38 | 4.08 | 4.08 | 3.79 | 3.21 | 3.21 |
| (MA) | 60.24 | 5.25 | 5.25 | 4.96 | 4.96 | 4.67 | 4.67 | 4.67 |
| (MA-co-VA) | 138.52 | 5.84 | 5.84 | 5.54 | 5.54 | 5.54 | 5.54 | 5.54 |
Table 5: Dye uptake studies of the graft copolymers vis-à-vis back bone.
Physico-chemico evaluation: The morphological transformations brought about by the graft copolymerization, modified the nature, properties and behavior of the fiber. These results obtained by XRD studies are supported by various physico-chemical tests. It was observed that when native fiber was compared with Hs-g-poly(MA) (Pg:60.24) and the highest grafted Hs-g-poly(MA-co-VA) (Pg:138.52) the properties of the fiber changed after graft copolymerization with the increase in Pg.
Moisture absorbance study: It is clear from Table 3, that graft co-polymerization of poly(MA) and other poly(vinyl) monomer chain onto H. sabdariffa had a great impact on the moisture absorbance behavior of the fiber. It has been observed that with increase in graft yield, there was significant decrease in the percentage of moisture absorbed in the graft copolymers. It was due to blocking of the sites vulnerable for moisture absorbance with hydrophobic poly (MA), poly (vinyl) monomer chains, thereby, transforming the fiber less sensitive to moisture [1-3,25].
Acid-base resistance study: The results obtained in Table 3 depict that acid-base resistance of the fiber increased with the increase in percent grafting. It could be accounted by the fact that poly (MA) and poly (vinyl) monomer chains grafted and covalently bound onto H. sabdariffa fiber (Hs-g-poly(MA-co-VA) had no weight loss on reactivity with 1 N HCl and 1N NaOH as compared to the free hydroxyl groups present in the ungrafted fiber with a weight loss of 55% and 43%, respectively. Therefore, the resistance in the fiber for strong acid and base was found to increase by the incorporation of poly (vinyl) chains on the active sites of the backbone after graft copolymerization [1-3,25].
Swelling behavior study: The swelling behavior studies were carried-out in different solvents like Water, MeOH, BuOH and DMF. It has been observed that H. sabdariffa fiber showed maximum swelling in Water (59%) followed by MeOH (46%), BuOH (38%) and DMF (30%). However, the swelling behavior of the graft copolymers followed the pattern: DMF > BuOH > Water > MeOH and the trend obtained had a direct correlation with the solubility parameters like solvent basicity, the molar ratio, hydrogen bond formation and the percentage grafting. The differential swelling behavior of the fibers could be justified depending upon the chemical nature and the property of the pendent groups (-COCH3, -COC4H9, -COOH,-CONH2 and -CN) that had different interactions with the solvent. Higher swelling in DMF and BuOH in grafted copolymers could be due to better interaction with the pendent groups that increases with increase in Pg. However, a reverse trend was found in the case of raw H. sabdariffa. Since, the cellulose is semi crystalline, polar polymer and miscible with in polar solvent due to hydrogen bond formation and imbibitions therefore, the raw fiber had higher swelling in water and MeOH followed by DMF and BuOH. Moreover, other factors like the fiber size, steric hindrance and temperature also influence the percentage of swelling [1-3,25] (Table 4).
Dye-uptake behavior: The dye uptake behavior of the graft copolymers vis-à-vis raw fiber was studied for seven consecutive hours to find out the effect of grafting on dye uptake. It is clear from Table 5 that the graft copolymers exhibited less dye uptake as compared to the raw fiber as it was found to be a function of Pg. It was observed that dye uptake decreases with increase in Pg. Cellulose is semi crystalline polymer that easily swells due to competitive processes of adsorption through hydrogen bonding and the scission of the internal hydrogen bonds between the amorphous molecules. Presence of free reactive sites like –OH and –CH2OH in raw fiber helped in the absorption of the dye. But, these sites get occupied with poly(MA) chains and poly(MA-co-VA) chains in the backbone that restrain dye uptake depending upon the Pg [1-3,25].
Conclusion
Grafting of poly (MA) and poly (MA-co-vinyl monomers) onto H. sabdariffa fiber in presence of Ceric ion initiator yielded novel regenerated graft copolymers. It is evident from the characterization by SEM, FTIR and XRD that the fiber properties were modified due to accumulation of the comonomers on the cellulosic backbone. Grafting of MA in binary mixture with VA yielded the highest graft yield (Pg:138.52). XRD confirmed the reduction in % Cr and C.I. with increase in Pg and it was supported by the results obtained by the evaluation of the properties of the grafted fiber. The grafted fibers were physico-chemico-thermally resistant and incorporated the features like hydrophobicity, miscibility in organic solvents that were desired for commercially and industrial applications. The use of this renewable waste bio-mass to obtain novel graft copolymers could be useful in insulator, packaging, aerospace and transportation. XRD is a simple advanced technique that could present the changes in nature, morphology of the fiber after graft copolymerization very easily.
References
- Kaith BS, Singha AS, Sharma SK (2004) Synthesis of Graft Copolymers of Binary Vinyl Monomer Mixtures and Flax Fiber using FAS-KPS Redox System. Int J Chem Sci 2: 37-43.
- Kaith BS, Singha AS, Sharma SK (2003) Graft Copolymerization of Flax Fibers with Binary Vinyl Monomer Mixtures and Evaluation of Swelling, Moisture absorbance and Thermal Behavior of the Grafted fibers. J Polym Mater 20: 195.
- Singha AS, Kaith BS, Kumar S (2004) Evaluation of Physical and Chemical Properties of FAS -KPS induced Graft Co-polymerization of Binary Vinyl monomer mixtures onto mercerized Flax. Int J Chem Sci 2: 472.
- Kaith BS, Singha AS, Dwivedi DK, Kumar S, Kumar D, Dhemeniya A (2003) Preparation of Polystyrene Matrix Based Composites using Flax-g-copolymers as Reinforcing agent and Evaluation of the Mechanical Behavior. Int J Plast Technol 7: 119-125.
- Kumar V, Bhardwaj YK, Rawat KP, Sabharwal S (2005) Radiation-induced grafting of vinylbezyltrimethylammonium chloride (VBT) onto cotton fabric and study of its anti-bacterial activities. Radiat Phys Chem 73: 175-182.
- Lee JS, Kumar RN, Rozman HD, Azemi BMN (2005) Pasting, swelling and solubility properties of UV initiated starch-graft-poly(AA). Food Chem 91: 203-211.
- Singh V, Tripathi DN, Tiwari A, Sanghi R (2006) Microwave synthesized chitosan-graft-poly(methyl-methacrylate): An efficient Zn2+ ion binder. Carbohyd Polym 65: 35-41.
- Pandey PK, Srivastava A, Tripathy J, Behari K (2006) Graft copolymerization of acrylic acid onto guar gum initiated by vanadium (V)–mercaptosuccinic acid redox pair. Carbohydr Polym 65: 414-420.
- Mishra A, Bajpai M (2005) Flocculation behavior of model textile wastewater treated with a food grade polysaccharide. J Hazard Mat 118: 213-217.
- Akkas P, Guven O (2000) Enhancement of uranyl ion uptake by prestructuring of acrylamide-maleic acid hydrogels. J Appl Polym Sci 78: 284-289.
- El-Sherbiny M, Abdel-Bary EM, Harding DRK (2006) Swelling characteristics and in vitro drug release study with pH- and thermally sensitive hydrogels based on modified chitosan. J Appl Polym Sci 102: 977-985.
- Qu X, Wirsen A, Albertsson AC (1999) Structural change and swelling mechanism of pH-sensitive hydrogels based on chitosan and D,L-lactic acid. J Appl Polym Sci 74: 3186-3192.
- Rudzinski WE, Dave AM, Vaishnav UH, Kumbar SG, Kulkarni AR, et al. (2002) Hydrogels as controlled release devices in agriculture. Des Mon Polym 5: 39-65.
- Huang J, Wang XL, Yu XH (2006) Solute permeation through the polyurethane-NIPAAm hydrogel membranes with various cross-linking densities. Desalination 192: 125-131.
- Ashish Chauhan, Balbir Singh (2011) X-Ray Powder Diffraction Studies to Accredit the Morphological Transformations in Hibiscus sabdariffa Graft-copolymers. International Journal of Polymer Analysis and Characterization 16: 319-328.
- Mishra MK, Tripathy AK (1981) Grafting vinyl monomers onto natural fibers. Graft copolymerization of methyl methacrylate onto wool and silk using peroxydiphosphate-tartaric acid redox system. Eur Polym J 17: 1225-1226.
- Monier M, Nawar N, Abdel-Latif D A (2010) Preparation and characterization of chelating fibers based on natural wool for removal of Hg(II), Cu(II) and Co(II) metal ions from aqueous solutions. J Haz Mater 184: 118-125.
- Okieimen FE, Idehen KI (1987) Note Graft Copolymerization of Methyl Methacrylate on Thiolated Holocellulose. J Macromol Sci: Part A Chem 24: 1381-1384.
- Sabaa MW, Mokhtar SM (2002) Chemically induced graft copolymerization of itaconic acid onto cellulose fibers. Polym Test 21: 337-343.
- Sharma VN, Daruwalla EH (1977) Thermal behavior of cotton grafted with vinyl monomers individually and in mixture compositions. J Appl Polm Sci21: 331-341.
- Taghizadeh MT, Khosravy M (2003) Kinetics and mechanism of graft copolymerization of vinyl monomers (Acrylamide, Acrylic acid and Methacrylate) onto starch by potassium Dichromate as redox initiator. Iran Polym J 12: 497-505.
- Zhu Z H, Ohgo K, Asakura T (2008) Preparation and characterization of regenerated Bombyx mori silk fibroin fiber with high strength. eXPRESS Polym Letters 2: 885-889.
- Thacker MS (1997) Malvaceae: A Dictionary of Indian Raw Materials & Industrial products. Vol. V, H-K, 80, CSIR Publisher, New Delhi.
- Junkasem J, Menges J, Supaphol P (2006) Mechanical properties of injection-molded isotactic polypropylene/roselle fiber composites. J Appl Polym Sci 101: 3291-3300.
- Chauhan A (2009) Synthesis and Evaluation of Physico-Chemico-Mechanical properties of polymer matrix based Composites using Graft copolymers of Hibiscus sabdariffa as reinforcing agents. PhD Thesis, Punjab Technical University, India.
- Kaith BS, Kalia S (2007) Synthesis and Characterization of Graft co-Polymers of Flax Fiber with Binary Vinyl Monomers. Int J Polym Analy Charact 12: 401-412.
- Fried JR (2005) Conformation, solution, and molecular weight In Polymer Science and Technol., (2ndEdn), 43, Upper saddle river, Prentice Hall Professional Technical Reference, NJ.
- Brandrup J, Immergut EH (1975) Polymer Handbook. (2ndEdn), Wiley Interscience, John Wiley and Sons, New York, 105.
- Ham G (1964) High Polymer. In: Copolymerization, Turner Alfrey, Interscience Publishers, 18: 939.
- Chauhan A, Kaith B (2011) Synthesis, characterization and chemical studies of Hibiscus sabdariffa-g-copolymers. Fibers and polymers 12: 1-7.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14857
- [From(publication date):
September-2012 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 10294
- PDF downloads : 4563