Research Article Open Access
Significance of Biotransformation in Drug Discovery and Development
| Selvan Ravindran*, Sudipta Basu, Prashant Surve, Ganesh Lonsane and Navya Sloka | |
| Department of Biotransformation, Drug Metabolism and Pharmacokinetics Unit, Sai Life Sciences Limited, International Biotech Park, Phase-II, Hinjewadi, Pune-411 057, India | |
| Corresponding Author : | Selvan Ravindran Department of Biotransformation Drug Metabolism & Pharmacokinetics Unit Sai Life Sciences Limited, International Biotech Park Phase-II, Hinjewadi, Pune-411 057, India Tel: +91 20 6674 3600 Fax: +91 20 6674 3645 E-mail: selvan.r@sailife.com |
| Received November 07, 2012; Accepted December 20, 2012; Published December 24, 2012 | |
| Citation: Ravindran S, Basu S, Surve P, Lonsane G, Sloka N (2012) Significance of Biotransformation in Drug Discovery and Development. J Biotechnol Biomaterial S13:005. doi: 10.4172/2155-952X.S13-005 | |
| Copyright: © 2012 Ravindran S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
Biotransformation is a process by which organic compounds are transformed from one form to another, aided by organisms such as bacteria, fungi and enzymes. Biotransformations are used as a valuable strategy to build molecules, similar to parent drug in the drug discovery programme. Biotransformations can also be used to synthesize compounds or materials, if synthetic approaches are challenging. Microbial biotransformations or microbial biotechnology are extensively used to generate metabolites in bulk amounts. Biotransformation approaches and synthetic methods in tandem provide a source for generating compounds around core structures, which can be screened for various biological activity studies. These studies help in screening and advancing the compounds, through various stages of drug discovery programme. Hence, biotransformation experiments can be effectively utilized to synthesize more compounds.
In the present study, a sulfonylurea urea drug was incubated with microsomal proteins to produce metabolites. Liquid chromatography is used to separate the formed metabolites and the parent drug. Fragmentation pattern obtained from the mass spectrum is utilized to identify the biotransformation site in the molecule, which is the metabolic soft spot. Identified new metabolites are structurally similar to parent drugs, implying the possibility of better therapeutic effects.
Few phase-I metabolites and phase-II metabolites are predicted with the help of liquid chromatography and mass spectrometry data. Synthesis of these identified, as well as predicted metabolites, and subjecting them to various pharmacological and toxicological studies will help to generate new materials with valuable therapeutic properties.
| Keywords |
| Biotransformation; Drug discovery; Mouse; Chromatography |
| Introduction |
| Developing a drug from the lab to the market is a massive task and a challenge [1]. From the pre-clinical stage onwards, great care is desired to advance the compounds [2]. Synthesis of compounds similar to that of the parent drug in the clinical trial will be a boon for the drug discovery programme. Failure of drugs [3] in the clinical trials will have to be supported by group of compounds, whose structure or properties are similar to that of the drug under clinical trials. Synthesized compound or materials have to go through biological assays, in vitro and in vivo drug metabolism, pharmacokinetic studies, pharmacological and toxicological studies. After performing all the studies, compounds are to be ranked for clinical trials, and the compound that satisfies all the chemical and biological assays will be further tested. In the event of failure, compounds with next best properties are to be tested [4]. Hence, synthesis of compounds with structure and properties similar to that of the parent compound in the clinical trial is necessary. |
| Biotransformation is one of the important experimental methods to identify compounds, whose structure and properties resembles the parent compound. During biotransformation, a particular drug is treated with microsomal proteins and screened for possible metabolites [5,6]. This helps to identify phase-I metabolites [7,8], that may result due to oxidation, reduction, dealkylation, epoxidation, dehalogenation, oxidative deamination, etc. Compounds upon treatment with hepatocytes or cytosol could lead to both Phase-I and Phase-II metabolites. The most common phase-II metabolites [9,10] are conjugate metabolites, which are directly formed from the parent drug, or from the phase-I metabolites. Glucuronide conjugates; sulfate conjugates, methylation, acetylation, sulfation, aminoacid conjugation and glutathione conjugation are considered as common phase-II metabolites. |
| Identifying the most probable structure of phase-I and phase- II metabolites using liquid chromatography and mass spectrometry [8-10], will assist in the synthesis of metabolites of various types. Synthesized metabolites can be subjected to various biological, DMPK, pharmacological and toxicological studies, which in turn, can lead to compounds with high activity and potency. There are many instances where metabolites have been developed as a drug. For example, desloratadine (clarinex) and fexofenadine (Allegra) were first identified as metabolites, and developed as drugs, later. Microbial and fungal biotransformation [11] was also used to produce metabolites. |
| Biotransformation also helps in optimizing the structure of the drug. For example, formation of glutathione adducts in a specific region of the molecule suggests the possibility of toxicity in the human system. This vital piece of information helps to perform necessary modifications in the identified site, and prevents the compound from resulting in toxicity [12]. In some instances, there may be a need for the compound to get metabolized, so that both bioavailability of the drug and clearance of the drug are well balanced. Hence, for some of the compounds which have poor clearance properties, there is a need to introduce certain functional groups in the compound, or make necessary modifications in the structure, so that a compound’s clearance property can be enhanced. For example formation of phase-II metabolites, such as glucuronide conjugates, are a better way to enhance the clearance property of the compound. |
| Materials and Methods |
| Chemicals and supplies |
| All the chemicals were purchased from Sigma Chemical Co., unless otherwise mentioned. |
| Human Liver microsomes |
| Pooled human liver microsomes (pool of 50 donors; Cat # 452165) were purchased from Xenotech. NADPH (Cat # N1630, 95% pure), potassium phosphate monobasic (Cat # P5655, >99% pure), potassium phosphate dibasic (Cat # P2222, 99% pure) and DMSO (Cat # D5879, >99.5% pure) were purchased from Sigma, Germany. |
| CYP enzymes activity was tested with positive controls, such as testosterone and 7-hydroxy coumarin. |
| Enzyme catalyzed reactions |
| The metabolic activity of microsomal preparations was determined in a reactive solution (1 ml total volume), made of the following components at their respective final concentrations: glyburide, 15 μM; microsomal protein, 1 mg/ml; 0.1 M potassium phosphate (pH 7.4). The contents were preincubated for 5 min at 37°C, and the reaction initiated by the addition of NADPH. The reaction was incubated for 30 min at the same temperature, terminated by the addition of ice cold acetonitrile. Samples were centrifuged at 10,000 g for 10 min, and the plates were discarded. 100 μl was transferred to HPLC vials and injected for analysis. |
| Instrument |
| Analytical separation of drug and its metabolites was achieved using a Agilent 1200 series HPLC system with quaternary pump (model G1311A), a column oven (model G1316A), an auto sampler (model G1367B), and a Diode Array Detector (DAD) (model G1316A). The analytical column utilized to separate drugs and metabolites was a reverse phase Zorbax 300 SB C18 150×4.5 mm, 5 μ analytical column. A variable flow gradient program was used to elute test compounds and its metabolites. A full scan mass analysis, from m/z 100 to 1000, was done to detect the parent and putative metabolites. Extracted ion current at m/z 510 is used to identify the hydroxylated metabolites of glyburide, and extracted ion current at m/z 412 is utilized to identify the metabolite due to loss of cyclohexyl ring. |
| Chromatographic conditions |
| Chromatographic separation was achieved using a Zorbax 300 SB C18 column (150×4.6 mm, 5 μ); the column temperature was maintained at 40°C. The mobile phase consisted of acetonitrile (solvent A) and water containing 0.1 % Formic acid, (solvent D). The gradient runs with a flow rate of 0.4 ml/min 90% A to 90% B, over 30 minutes. The elution flow from HPLC was introduced into the Q-Trap spectrometer ionization source, through an ESI interface. |
| Mass spectrometric conditions |
| The identities of the primary in vitro glyburide metabolites were confirmed using Q-TRAP-MS/MS (API4000 Q-TRAP, Applied Biosystems), operated in positive ion, conducted using Electro Spray Ionization (ESI) mode. Enhanced Mass Scan (EMS) and Enhanced Product Ion (EPI) scans were used to elucidate the structure of metabolites. The LC/MS/MS parameters used are as follows: Collision energy used for EMS scan is 9 V and for EPI scan is 36 V, with a collision energy spread of 5. De cluster potential is 102 V. Curtain gas was set to 30 psi and CAD gas was set to medium. Ion spray voltage was 5400 volts. Ion source gas 1 and 2 were 55 psi and 60 psi, respectively, and temperature was tuned to 550°. |
| Results |
| Metabolism of glyburide in mouse liver microsomes |
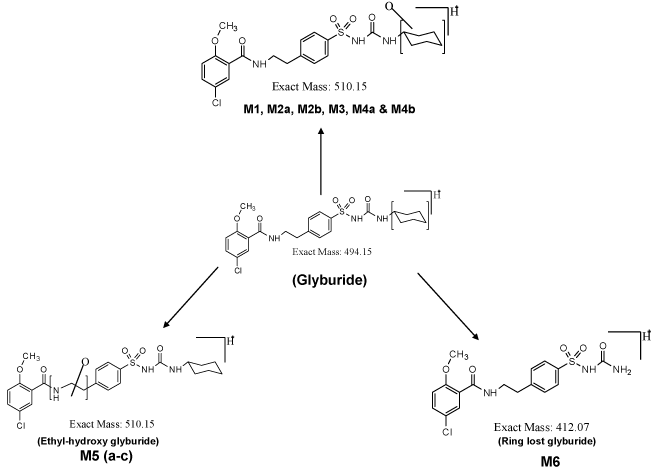
| The PDA chromatogram for the metabolites of glyburide is shown in supplementary data of figure 1. Incubation of glyburide with mouse liver microsomes for 60 minutes results in the formation of metabolites. Metabolites M1, M2a, M2b, M3 and M4 are monohydroxylated metabolites. Mono-oxygenation occurs in the cyclohexyl group of the glyburide molecule. Metabolite M1 elutes at the retention time of 22.9 minutes. Metabolite M2a and M2b are closely eluting metabolites at the retention times of 23.4 and 23.5 minutes, respectively. Metabolite M3 was detected at 23.77 minutes and metabolite M4 was identified at 24.7 minutes. Earlier studies using liquid chromatography and mass spectrometry had confirmed the structure for these metabolites, when the incubation of glyburide with human or mouse liver microsomes was performed for 30 mins [5,8]. In the present study, incubation was carried out for 60 minutes, and similar metabolites were identified. |
| Metabolite M5 elutes at the retention time of 26.4 minutes. Metabolite M5 has different structure, where hydroxylation is expected to occur at amino ethyl group (-NH2-CH2-CH2) of the molecule, which is flanked between carbonyl and benzene ring of the molecule. Formation of metabolite M6 is due to elimination of the cyclohexyl group. The structure of this particular metabolite is confirmed by our earlier study, where different scan modes such as neutral loss scan, precursor ion scan, Enhanced Mass Scan coupled with Enhanced Product Ion scan were utilized to identify the structure of metabolites. |
| Total ion chromatogram and extracted ion chromatogram for glyburide and metabolites |
| Total ion chromatogram (Supplementary data of Figure 2a) is similar to the UV chromatogram obtained from the PDA detector (Supplementary data of Figure 1). Parent drug glyburide appears as an intense peak at the retention time of 28.4 minutes. Intensities of other metabolites are imprecise in the total ion chromatogram. Molecular mass m/z 510 due to monohydroxylated metabolite is specifically extracted from the total ion current, and shown in the supplementary data of figure 2b, as extracted ion chromatogram. All the six monohydroxylated metabolites identified in the PDA detector was identified in the extracted ion chromatogram at similar retention times. Metabolite M6 due to the loss of cyclohexyl ring is extracted from the total ion current at m/z 412. This metabolite elutes at the retention time of 22.7 minutes. |
| Discussion |
| Incubation of mouse liver microsomes with parent drug glyburide results in monohydroxylated metabolites [5], and ring loss metabolite [6]. Structures of the cyclohexyl monohydroxy glyburide have been characterized in the previous work [5,8]. For example: 4-trans-hydroxy cyclohexyl glyburide, 4-cis-hydroxy cyclohexyl glyburide, 3-trans-hydroxy cyclohexyl glyburide, 3-cis-hydroxy cyclohexyl glyburide and 2-trans-hydroxy cyclohexyl glyburide have been synthesized as materials, and their retention time were compared with the retention time of metabolites found in the incubation mixture. Thus, the structure of the formed metabolites in the incubation mixture is confirmed. Among the synthesized materials, two of the materials, i.e. 4-trans-hydroxy cyclohexyl glyburide and 3-trans hydroxyl cyclohexyl glyburide exhibit hypoglycemic activity; however, its activity is less than that of the parent drug glyburide [13]. This suggests the importance of identification of metabolites, and testing those metabolites for activity and potency in animal models. For example, popular drugs such as Acetaminophen, Oxazepam, and Cetrizine, etc. in the market are identified as metabolites, and later developed as a drug. Similarly, many metabolites are developed as a better drug than those of the parent drug [14]. In this context, identification of metabolites, prediction of important metabolites and synthesis of metabolites, and subjecting those metabolites for preclinical studies gains importance for the following reasons. |
| (1) To find out whether the metabolite has better potency and activity than the parent drug. |
| (2) To have better understanding about the toxicological properties of the metabolites, and |
| (3) To find whether the quantity of formed metabolites are in accordance with the FDA (Food and Drug Administration) guidelines. |
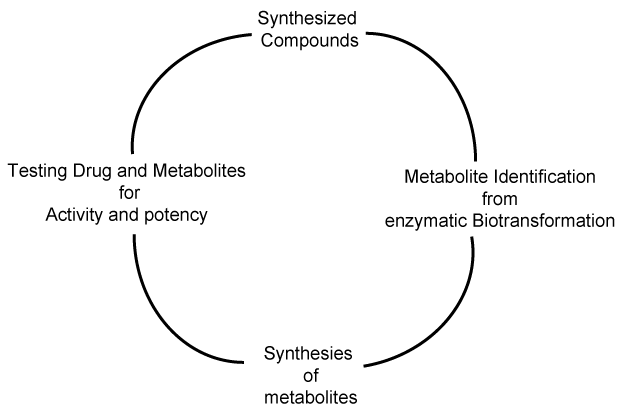
| Importance of metabolite identification in drug discovery program is shown as a cycle in figure 1, where the typical cycle starts with the synthesis of new compound or material. The synthesized material is subjected to enzymatic biotransformation study, where the major and minor metabolites are identified using liquid chromatography and mass spectrometry. Nuclear magnetic resonance spectrometry can be utilized to characterize the structure of a compound, if a particular metabolite is found in significant quantities. Figure 2 shows the putative metabolites that can be formed, based on the liquid-chromatography and mass spectrometry studies. Metabolites M1 to M4 have been synthesized as materials and its structure confirmed, based on the LC-MS/MS studies [5]. Phase-I metabolite M5a, M5b, M5c and M6 have not been synthesized as materials, so far [8]. Metabolite identification studies provide us an insight to develop new materials, whose properties can be pharmacologically very important. |
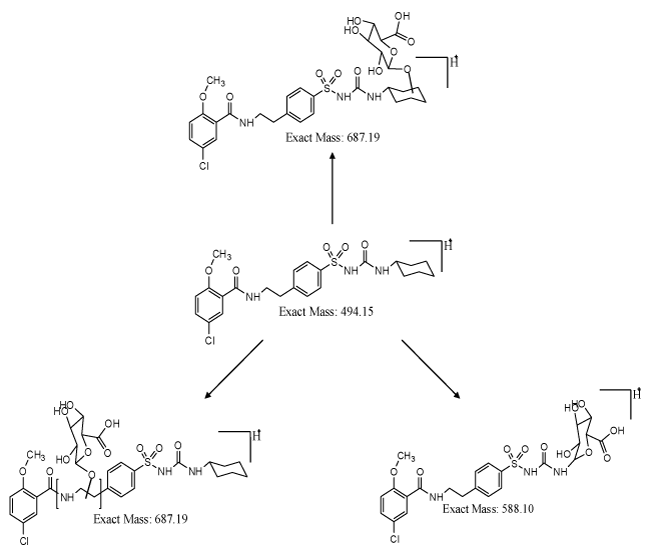
| Formation of Phase-II metabolites gains significance from the pharmacokinetics and pharmacological point of view. If the drug or compound is highly hydrophobic, removal of the drug after consumption by human or animal is by the process of metabolism. In the metabolic process, hydrophobic drug is converted to hydrophilic drug and cleared from the system. This process aids in diminution of the toxicity of the material, if the material stays for longer time in the system. In case of glyburide, formation of hydroxylated metabolites gains significance, which is eliminated from the system. In certain cases, elimination process will not be feasible, even after phase-I metabolism. In such instances, formed phase-I metabolites need to be converted to phase-II metabolites, where hydroxylated metabolites have to form conjugates with glucuronides, glutathione, sulfates or amino acids. The possible glucuronide conjugates for glyburide is shown in figure 3. Phase-I metabolite, i.e., hydroxyl cyclohexyl glyburide and ethyl hydroxyl glyburide can be converted to respective O-glucuronide conjugates, resulting in more hydrophilic compounds, and assisting the clearance of compound from the system. These O-glucuronide conjugates were highly polar, and can be cleared from the system at a faster rate than the parent metabolites. Synthesis of conjugates will help to understand more about the pharmacological properties of the parent drug and its conjugates. Metabolite M6 can result in N-glucuronide conjugate, which can be pharmacologically important and significant. There are instances where formation of amine metabolite could be toxic, in such cases, formation of N-glucuronides facilitating the elimination of drug from the system gains importance. Thus, the synthesis of both phase-I and phase-II metabolites based on the liquid chromatography and mass spectrometry data, and subjecting those metabolites for pharmacological and toxicological studies are very important in drug discovery programme. |
| Conclusions |
| Metabolites formed from enzymatic biotransformation of compounds possess structures similar to that of the parent compound. Identification of the tentative structure of metabolites, using liquid chromatography and mass spectrometry, helps to design and synthesize new molecules or materials similar to that of the parent compound. Subjecting the synthesized new compounds for various in vitro and in vivo assays will help to enhance the pharmacological properties of the compounds. Thus, the synthesized metabolites can be a compound or material, whose properties might be similar to that of the parent drug, and can serve as an ideal back up compound for parent drug in clinical trials. |
| Acknowledgements |
| The authors thank Sai Life Sciences Limited, India for financial support and providing necessary facility to carry out this work successfully. We thank Dr. Hitesh Patel, Sai Life Sciences Limited, for valuable discussions and intellectual input. |
References
- Vangala S, Pinjari J, Patole P, Ravindran S, Gangal R, et al. (2012) Translational drug discovery research: integration of medicinal chemistry, computational modeling, pharmacology, ADME, and toxicology. Encyclopedia of drug metabolism and interactions 1-54.
- Department of Health and Human Services (2008) Guidance for industry. Safety testing of drug metabolites. Food and Drug Administration, Center for Drug Evaluation and Research 1-11.
- Arrowsmith J (2011) Trial watch: phase III and submission failures: 2007-2010. Nat Rev Drug Discov 10: 87.
- Fura A (2006) Role of pharmacologically active metabolites in drug discovery and development. Drug Discov Today 11: 133-142.
- Ravindran S, Zharikova OL, Hill RA, Nanovskaya TN, Hankins GD, et al. (2006) Identification of glyburide metabolites formed by hepatic and placental microsomes of humans and baboons. Biochem Pharmacol 72: 1730 - 1737.
- Ravindran S, Honrao C, Sahu R, Basu S, Basit A, et al. (2011) Determining quantity of metabolites without synthetic standards: An approach using LC-PDA-MS. International Journal of Chemical and Analytical Science 2: 1219-1221.
- Ravindran S, Honrao C, Sahu R, Basit A, Madireddy S, et al. (2011) Optimal use of mass spec scan modes to identify an unknown metabolite. Drug Invention Today 3: 259-261
- Ravindran S, Basu S, Gorti SKK, Surve P, Sloka N (2012) Metabolic profile of glyburide in human liver microsomes using LC-DAD-Q-TRAP-MS/MS. Biomed Chromatogr
- Yu LJ, Chen Y, Deninno MP, O’Connell TN, Hop EC (2005) Identification of novel glutathione adduct of diclofenac, 4’-hydroxy-2’glutathion-deschloro-diclofenac upon incubation with human liver microsomes. Drug Metab Dispos 33: 484-488.
- Ogilvie BW, Zhang D, Li W, Rodrigues D, Gipson AE, et al. (2006) Glucuronidation converts gemfibrozil to a potent, metabolism dependent inhibitor of CYP2C8: Implications for drug-drug interactions. Drug Metab Dispos 34: 191-197.
- Manzoni M, Rollini M (2002) Biosynthesis and biotechnological production of statins by filamentous fungi and application of these cholesterol-lowering drugs. Appl Microbiol Biotechnol 58: 555-564.
- Drayer DE (1976) Pharmacologically active drug metabolites: therapeutic and toxic activities, plasma and urine data in man, accumulation in renal failure. Clin Pharmacokinet 1: 426-443.
- Rydberg T, Jonsson A, Roder M, Melander A (1994) Hypoglycemic activity of glyburide (glibenclamide) in humans. Diabetes Care 17: 1026-1030.
- Fura A, Shu YZ, Zhu M, Hanson RL, Roongta V, et al. (2004) Discovering drugs through biological transformation: role of pharmacologically active metbolites in drug discovery. J Med Chem 47: 4339-4351.
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 29672
- [From(publication date):
specialissue-2013 - Jan 07, 2026] - Breakdown by view type
- HTML page views : 24525
- PDF downloads : 5147
