Research Article Open Access
Optimization of Lipase Catalyzed Synthesis of Nonyl Caprylate using Response Surface Methodology (RSM)
Syamsul Kamar MW1*, Salina MR1, Siti Salhah O1, Hanina MN1 and Mohd Basyaruddin AR21Faculty of Science and Technology, Universiti Sains Islam Malaysia (USIM), Bandar Baru Nilai, 71800 Nilai, Negeri Sembilan, Malaysia
2Faculty of Science, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia
- Corresponding Author:
- Syamsul Kamar MW
Faculty of Science and Technology
Universiti Sains Islam Malaysia (USIM)
Bandar Baru Nilai, 71800 Nilai, Negeri Sembilan, Malaysia
Tel: +6013-2101647
Fax: +606-7987010
E-mail: syams_kamar@yahoo.com
Received date: April 19, 2011; Accepted date: June 22, 2011; Published date: June 24, 2011
Citation: Syamsul Kamar MW, Salina MR, Siti Salhah O, Hanina MN, Mohd Basyaruddin AR (2011) Optimization of Lipase Catalyzed Synthesis of Nonyl Caprylate using Response Surface Methodology (RSM). J Biotechnol Biomaterial 1:106. doi:10.4172/2155-952X.1000106
Copyright: © 2011 Syamsul Kamar MW, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
Nonyl caprylate, a citrus and rose flavor is short-chain ester with fruity notes is widely used in food, cosmetic and pharmaceutical industries. Traditionally, flavor esters are produced by chemical method or extracted from natural sources. However, with the steadily growing demand for natural flavor compounds, the biosynthesis of such esters by lipase under mild conditions has been receiving much attention for producing these valuable products. In this study, enzymatic synthesis of nonyl caprylate in solvent free system, was successfully optimized via Response Surface Method (RSM) based on 5-level, 4-variable of Central Composite RotaTable Design (CCRD). The parameters were reaction time (3-8 hours); reaction temperature (30-50°C); amount of enzyme (10-20 %, w/w) and shaking speed (100-200 rpm). The optimum condition derived via RSM for the reaction was reaction time of 6.6 hours, reaction temperature of 30.08ºC, enzyme amount of 20 % (w/w) and shaking speed of 128.7 rpm. The actual experimental yield was 90.91% under the optimum condition, which compared well with the maximum predicted value of 91.33%. Comparison of predicted and experimental values reveals good correspondence between them, implying that empirical models derived from RSM can be used to adequately describe the relationship between the factors and response in the synthesis of Nonyl Caprylate.
Keywords
Nonyl caprylate; Flavor ester; Lipase; Optimizatipn; Response surface method
Introduction
Since the end of the last century, people are becoming more concerned about their health. Natural foods are a common requirement nowadays, which include natural ingredients. This suggests that natural flavor compounds could no more be obtained by extraction from plants alone because of the great demand. The main aim of the scientific community is not only to create natural flavors, but also to develop new technologies. This includes three principal techniques of biosynthesis that can be distinguished in the following ways: (1) use of enzymes, (2) use of microorganisms, and (3) plant cells and culture of tissues. Among those, application of enzymes is the most frequently used technique of biosynthesis [9].
Flavor esters have been generally produced by lipases from various sources in organic solvents. But solvent toxicity and high production costs are problems for most reactions. To facilitate these, solvent free system offers some advantages, where the absence of solvents facilitates downstream processing, thus offering significant cost saving and minimizing environmental impact. The number of publications referring to enzymatic synthesis of flavor esters in non-conventional media, particularly in the presence of solvents, is numerous [1,9,12]. On the other hand, the number of articles discussed enzymatic synthesis in solvent free systems is considerably low [10].
Biotechnological production of flavor esters with lipases has recently received greater consideration with respect to the traditional methods and is undergoing a rapid development. This is due to the mild operating conditions, the higher degree of purity of the products and their acceptability in the food industries. An understanding of their biosynthesis will enable us to control the production of flavor esters in food products. The use of immobilized lipases is preferred due to its ease of handling, easy enzyme-product separation, and reusability [12].
Optimization of reaction process is very important in enzymatic synthesis to improve the reaction performance. However, most of the reported findings are based on the conventional study where only one parameter was varied at any one time, resulting in only an ‘apparent’ set of optimal conditions [1,9,12]. Understanding and modeling of both conventional and interactive effects of important parameters are essential in order to obtain a high performance synthesis [7].
A statistical based technique commonly used for this purpose is Response Surface Method (RSM), where the reactions are varied simultaneously in a suiTable program manner to generate data for the development of empirical models. This technique of RSM is a powerful tool to determine the optimum operating conditions necessary to scale up the process and to reduce the number of and cost of experiments. RSM has been successfully applied to the optimization of enzymatic syntheses of various fine chemical products [7].
Therefore, the purpose of this research is to study the optimization process of green synthesis of flavor esters via lipase-catalyzed reactions in solvent free system via statistical approach of Response Surface Methodology (RSM). Nonyl caprylate which is known as the main component in citrus and rose flavor is the targeted flavor ester in this investigation. It is necessary to identify the important factors that affect the performance of the process in small scale system, so that a sui Table approach of scaling-up and a design model could be proposed prior to commercialization.
Materials and Methods
Materials
Novozym 435 as 10,000 PLU (from Candida antarctica lipase immobilized onto macroporous acrylin resin) was received from Novo Nordisk (Denmark). Nonanol (purity, 98 %) and caprylic acid (purity, 97%) were obtained from Merck (Germany). All other reagents were of analytical grade and used as received.
General enzymatic synthesis
The general reaction system consisted of nonanol and caprylic acid (1:1) and 5 % of enzyme (w/w) were mixed in a screw-capped vial. The mixture was incubated at 37°C using a horizontal waterbath shaker. The shaking speed was set at 150 rpm and the reaction mixture was continuously reacted for 12 hours. After that, the following conditions were generated using the software’s numerical optimization function.
Analysis of reaction product
Determination of the percentage conversion of ethyl valerate (%): The percentage conversion (%) of Nonyl Caprylate was measured by determining the remaining unreacted fatty acids in the reaction mixture by titration with 1.0 M NaOH in an automatic titrator (Methrom, Switzerland). All the samples were assayed in triplicate and the experiment was repeated twice.
Equation 1:
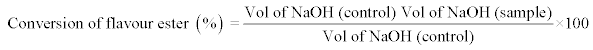
Experimental design: A five-level, four-factor Central Composite RotaTable Design (CCRD) was employed, requiring 30 experiments. The fractional factorial design consisted of sixteen factorial points, eigth axial points and six centre points. The variables and their respective levels are presented in Table 1. Table 2 represents the actual experiments carried out for developing the model. The data obtained were fitted to a second-order polynomial equation 2.
| Standard | A (hour) | B % (w/w) | C (°C) | D (rpm) | Actual (%) | Predicted (%) |
|---|---|---|---|---|---|---|
| 1 | 3 | 10 | 30 | 100 | 81.337 | 82.116 |
| 2 | 8 | 10 | 30 | 100 | 85.722 | 85.976 |
| 3 | 3 | 20 | 30 | 100 | 89.603 | 89.114 |
| 4 | 8 | 20 | 30 | 100 | 90.361 | 91.112 |
| 5 | 3 | 10 | 50 | 100 | 84.115 | 83.777 |
| 6 | 8 | 10 | 50 | 100 | 85.785 | 86.013 |
| 7 | 3 | 20 | 50 | 100 | 89.783 | 89.731 |
| 8 | 8 | 20 | 50 | 100 | 89.898 | 90.154 |
| 9 | 3 | 10 | 30 | 200 | 86.937 | 86.510 |
| 10 | 8 | 10 | 30 | 200 | 88.716 | 89.119 |
| 11 | 3 | 20 | 30 | 200 | 89.687 | 89.810 |
| 12 | 8 | 20 | 30 | 200 | 90.441 | 90.607 |
| 13 | 3 | 10 | 50 | 200 | 87.210 | 86.811 |
| 14 | 8 | 10 | 50 | 200 | 87.528 | 87.846 |
| 15 | 3 | 20 | 50 | 200 | 89.543 | 89.117 |
| 16 | 8 | 20 | 50 | 200 | 88.817 | 88.339 |
| 17 | 0.5 | 15 | 40 | 150 | 83.149 | 83.829 |
| 18 | 10.5 | 15 | 40 | 150 | 87.720 | 86.861 |
| 19 | 5.5 | 5 | 40 | 150 | 84.756 | 84.412 |
| 20 | 5.5 | 25 | 40 | 150 | 91.688 | 89.069 |
| 21 | 5.5 | 15 | 20 | 150 | 90.463 | 89.748 |
| 22 | 5.5 | 15 | 60 | 150 | 88.556 | 89.092 |
| 23 | 5.5 | 15 | 40 | 50 | 88.019 | 87.389 |
| 24 | 5.5 | 15 | 40 | 250 | 89.467 | 89.918 |
| 25 | 5.5 | 15 | 40 | 150 | 88.698 | 89.069 |
| 26 | 5.5 | 15 | 40 | 150 | 89.657 | 89.069 |
| 27 | 5.5 | 15 | 40 | 150 | 88.388 | 89.069 |
| 28 | 5.5 | 15 | 40 | 150 | 89.207 | 89.069 |
| 29 | 5.5 | 15 | 40 | 150 | 88.966 | 89.069 |
| 30 | 5.5 | 15 | 40 | 150 | 89.499 | 89.069 |
Table 1: Coded and actual levels of variables for design of experiment
| Coded values of variables | |||||||
| Factor | Name | Unit | -2 | -1 | 0 | 1 | 2 |
| A | Time | hour | 0.5 | 3 | 5 | 8 | 10.5 |
| B | Enzyme Amount | % (w/w) | 5 | 10 | 15 | 20 | 25 |
| C | Temperature | °C | 20 | 30 | 40 | 50 | 60 |
| D | Shaking Speed | rpm | 50 | 100 | 150 | 200 | 250 |
aStudy type, response surface; No. of experiments, 30; design, CCRD; response, Y1, name, flavour ester, unit, % conversion.
Table 2: Design matrix of the actual experiments carried out for developing the model.
Equation 2:
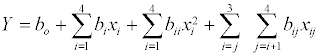
where Y= % conversion of flavour ester, b0, bi, bii and bij are constant coefficients and xi are the uncoded independent variables. Subsequent regression analysis, Analysis Of Variance (ANOVA) and response surfaces were performed using Design Expert Software (version 7.1.6) from Stat-Ease (Minneapolis, MN). Optimal reaction parameters for maximum conversion were generated using the software’s numerical optimization function.
Result and Discussion
Model fitting and ANOVA
The coefficients of the empirical model and their statistical analysis, evaluated using Design Expert Software, are presented in Table 3-5. The model F-value of 24.74 with a ‘Prob > F ’ value of 0.0001 implied that the model was significant at the 1 % confidence level. The high coefficient of determination (R2= 0.9585) of the model indicated the suitability of the model for adequately representing the real relationship among the parameters studied. A high value of R2 (>0.950) has been also reported by [13] for the lipase-catalysed synthesis of isoamyl isobutyrate and by [6] for the enzymatic optimization of propylene glycol monolaurate by direct esterification. In this study, quadratic model was shown to be the most significant model due to the low value of probability (P=0.0001) and high value of coefficient determination (R2=0.9585). Similar quadratic response models have been reported by [2,3] in the optimization of lipase-catalyzed synthesis of biodiesel (soybean oil methyl ester) and kojic acid monolaurate, respectively. The model indicates the significant terms were observed for linear (A and C), quadratic (C) and interactive effect (AC) according to the value of ‘Prob > F’ < 0.050. The final equation was derived in terms of coded factors for the synthesis of ethyl valerate as shown in Equation (3):
| Source | Sum of squares | Degrees of freedom | Mean Square | F-value | Prob > F |
|---|---|---|---|---|---|
| Model | 155.92 | 14 | 11.14 | 24.74 | <0.0001 |
| Residual | 6.75 | 15 | 0.45 | ||
| Lack of fit | 5.59 | 10 | 0.56 | 2.41 | 0.1720 |
| Pure error | 1.16 | 5 | 0.23 | ||
| Total | 162.67 | 29 |
aSignificance at ‘Prob>F’ is <0.0500
Table 3: Statistical analysis: ANOVA
| Std. Dev. | 0.67 |
| Mean | 88.12 |
| R-squared | 0.9585 |
| Adj-R-Squared | 0.9198 |
| Pred-R-Squared | 0.7917 |
| Adeq Precision | 20.419 |
Table 4: Statistical analysis: regression analysis
| Factor | Coefficient Estimate | Prob > F |
|---|---|---|
| Intercept | 89.07 | <0.0001 |
| A-Time | 0.76 | <0.0001 |
| B-Temperature | 1.86 | <0.0001 |
| C-Enzyme Amount | -0.16 | 0.2496 |
| D-Shaking Speed | 0.63 | 0.0003 |
| AB | -0.45 | 0.0164 |
| AC | -0.39 | 0.0331 |
| AD | -0.30 | 0.0936 |
| BC | -0.25 | 0.1590 |
| BD | -0.91 | <0.0001 |
| CD | -0.33 | 0.0698 |
| A2 | -0.93 | <0.0001 |
| B2 | -0.23 | 0.0875 |
| C2 | 0.088 | 0.5042 |
| D2 | -0.10 | 0.4298 |
Table 5: Statistical analysis: coefficient of models
Equation 3:
Y = +89.07 + 0.76A+1.86B - 0.16C +0.63D - 0.45AB - 0.39AC-0.30AD -0.25BC -0.91BD -0.33CD-0.93A2 -0.23B2 + 0.088C2-0.10D2
where A is the time; B is the amount of enzyme; C is the temperature; D is the shaking speed.
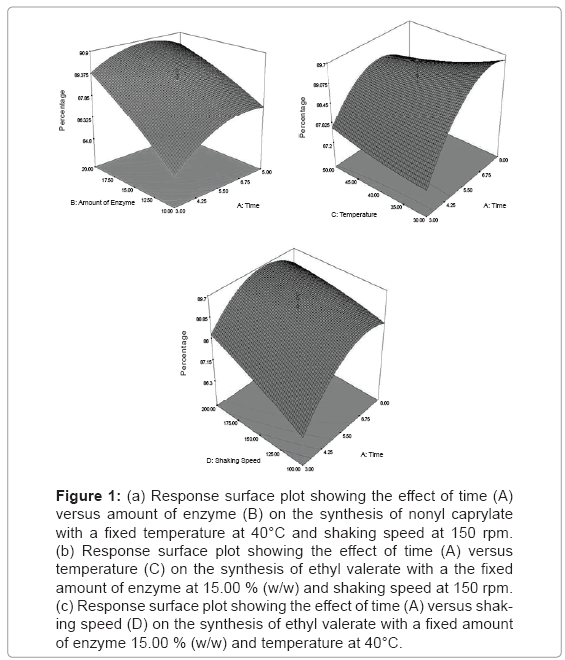
Response surface plot: Equation (3) was then used to facilitate plotting of the response surfaces. Two parameters were plotted at one time on the X1 and X2 axes, respectively, with another parameter set at their centre point value (coded level:0). Figure 1a–c illustrate the response surface plots as a function of time (A) versus the amount of enzyme (B), temperature (C) and shaking speed (D), respectively. Generally, all figureureures show the percentage conversion of nonyl caprylate was increased with increasing the temperature, amount of enzyme and shaking speed of approximately 85.00% conversion to nonyl caprylate. However, it was found that the percentage conversion was sligthly decreased with increasing the temperature from 35.0 to 50.00°C at constant time 8 hours. An increment in the reaction temperature improved the substrate solubility and dissociation, but a decrease in the binding equilibrium, leading to unfavorable esterification conditions [4]. Furthermore, the use of low temperature is beneficial in that, power costs can be reduced and enzyme stability can be preserved during prolonged operation. The similar finding were also reported by [13] and [4], for the lipozyme-catalyzed synthesis of amino acid esters and isoamyl isobutyrate, respectively. In enzymatic reaction, organic solvent was usually used to improve the solubility of the substrates and enhance the production yield. In order to reduce the cost and solvent toxicity, a solvent free system was applied in this study. The result shows that at optimized variables of temperature, amount of enzyme and shaking speed, it could lead to produce high conversion of flavor ester even in a shorter time period. This is contrast with the work that has been done by [10] whereby the equilibrium conversion of ethyl valerate using immobilized Staphylococcus stimuli in the presence of organic solvent was only acheived at 18 hours of incubation period. On the other hand, the use of solvents free system also lead us to purify the product easily without any toxicity and inflammability problems.
Figure 1: (a) Response surface plot showing the effect of time (A) versus amount of enzyme (B) on the synthesis of nonyl caprylate with a fixed temperature at 40°C and shaking speed at 150 rpm. (b) Response surface plot showing the effect of time (A) versus temperature (C) on the synthesis of ethyl valerate with a the fixed amount of enzyme at 15.00 % (w/w) and shaking speed at 150 rpm. (c) Response surface plot showing the effect of time (A) versus shaking speed (D) on the synthesis of ethyl valerate with a fixed amount of enzyme 15.00 % (w/w) and temperature at 40°C.
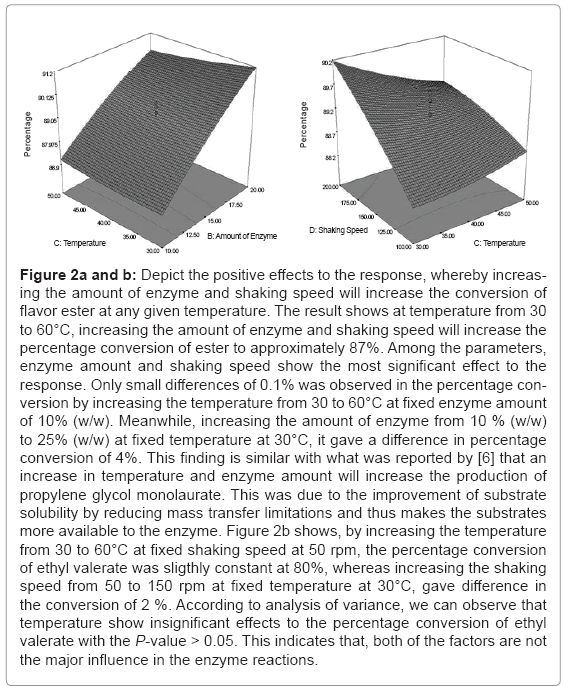
Figure 2a and b depict the positive effects to the response, whereby increasing the amount of enzyme and shaking speed will increase the conversion of flavour ester at any given temperature. The result shows at temperature from 30 to 60°C, increasing the amount of enzyme and shaking speed will increase the percentage conversion of ester to approximately 87%. Among the parameters, enzyme amount and shaking speed show the most significant effect to the response. Only small differences of 0.1% was observed in the percentage conversion by increasing the temperature from 30 to 60°C at fixed enzyme amount of 10% (w/w). Meanwhile, increasing the amount of enzyme from 10 % (w/w) to 25 % (w/w) at fixed temperature at 30°C, it gave a difference in percentage conversion of 4%. This finding is similar with what was reported by [6] that an increase in temperature and enzyme amount will increase the production of propylene glycol monolaurate. This was due to the improvement of substrate solubility by reducing mass transfer limitations and thus makes the substrates more available to the enzyme. Figure 2b shows, by increasing the temperature from 30 to 60°C at fixed shaking speed at 50 rpm, the percentage conversion of ethyl valerate was sligthly constant at 80%, whereas increasing the shaking speed from 50 to 150 rpm at fixed temperature at 30°C, gave difference in the conversion of 2%. According to analysis of variance, we can observed that temperature show insignificant effects to the percentage conversion of ethyl valerate with the P-value > 0.05. This indicates that, both of the factors are not the major influence in the enzyme reactions.
Figure 2a and b: Depict the positive effects to the response, whereby increasing the amount of enzyme and shaking speed will increase the conversion of flavor ester at any given temperature. The result shows at temperature from 30 to 60°C, increasing the amount of enzyme and shaking speed will increase the percentage conversion of ester to approximately 87%. Among the parameters, enzyme amount and shaking speed show the most significant effect to the response. Only small differences of 0.1% was observed in the percentage conversion by increasing the temperature from 30 to 60°C at fixed enzyme amount of 10% (w/w). Meanwhile, increasing the amount of enzyme from 10 % (w/w) to 25% (w/w) at fixed temperature at 30°C, it gave a difference in percentage conversion of 4%. This finding is similar with what was reported by [6] that an increase in temperature and enzyme amount will increase the production of propylene glycol monolaurate. This was due to the improvement of substrate solubility by reducing mass transfer limitations and thus makes the substrates more available to the enzyme. Figure 2b shows, by increasing the temperature from 30 to 60°C at fixed shaking speed at 50 rpm, the percentage conversion of ethyl valerate was sligthly constant at 80%, whereas increasing the shaking speed from 50 to 150 rpm at fixed temperature at 30°C, gave difference in the conversion of 2 %. According to analysis of variance, we can observe that temperature show insignificant effects to the percentage conversion of ethyl valerate with the P-value > 0.05. This indicates that, both of the factors are not the major influence in the enzyme reactions.
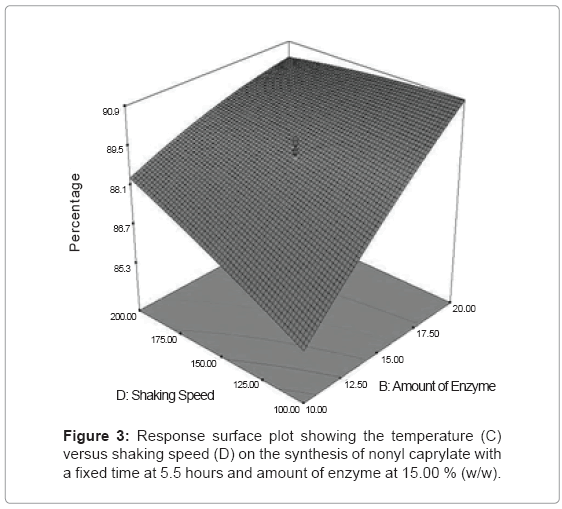
Figure 3 represents the effect of amount of enzyme (C) versus shaking speed (D) at fixed time, 5.5 hours and temperature, 40°C. At high amount of enzyme (20% (w/w) and shaking speed (200 rpm), maximum conversion of more than 90% was observed. Amount of enzyme shows better influence compared to shaking speed. By referring the 3D surface graph, an increase in shaking speed from 50 rpm to 200 rpm at amount of enzyme 10% (w/w), gave the difference in percentage conversion of only 3%. However, by increasing the amount of enzyme from 10% (w/w) to 20% (w/w) at the fixed shaking speed 50 rpm, it gave the difference in percentage conversion of 5%. This was due to the increase in acceleration of enzyme movement which resulted in high reaction rate between the substrates and enzyme molecules. Higher enzyme amount also increased the formation of acyl-enzyme intermediate to produce the product, which was in agreement with the results by [5,11] on the synthesis of palm-based wax esters using Lipozyme RM IM.
Optimization of reaction
The optimum condition for the lipase-catalyzed synthesis of nonyl caprylate was predicted using the optimization function of the Design Expert Software. Table 6 shows the optimum condition of their experimental and predicted values. Comparison of experimental and predicted values revealed good correspondence between them, implying that empirical model derived from RSM can be used to adequately describe the relationship between the factors and response in lipase-catalyzed synthesis of ethyl valerate. From an economic viewpoint, it is desirable to choose the lowest possible reaction time, temperature, amount of enzyme and shaking speed for practical esterification of nonyl caprylate. The optimum conditions can be used for future upscale synthesis of nonyl caprylate. Novozym 425 can work well up to temperature of 60°C as reported by the most manufacturer. Many reactions were undertaken at temperature range 30-70°C to test for its performance [7]. Meanwhile a temperature of 30°C was sufficient to produce high yield of over 80% in lipase-catalyzed synthesis of nonyl caprylate. From economic standpoint, it would be favorable to use the minimum time and enzyme amount to attain maximum conversion. If it was necessary to complete the synthesis with the highest percentage conversion without concern for cost, the amount of enzyme should be considered first, and then the other factors could be maximized [5]. All optimum conditions can be used to produce high % conversion of ethyl valerate.
Conclusion
Comparison of predicted and experimental values revealed a good correspondence between them, implying that empirical models derived from RSM can be used to adequately describe the relationship between factors and response in lipase-catalyzed synthesis of nonyl caprylate. This model can be used to predict flavour ester conversion under any given conditions within the experimental range. We have demonstrated that optimum synthesis of flavour esters can be successfully predicted by RSM.
Acknowledgements
This project was finance by a grant from FRGS-FST-06-50408, Malaysia.
References
- Chang SW, Shaw JF, Yang CK, Shieh CJ (2007) Optimal Continuous Biosynthesis of Hexyl Laurate by a Packed Bed Bioreactor. Process Biochem 42: 1362-1366.
- Shieh CJ, Liao HF, Lee CC (2003) Optimization of Lipase-catalysed Biodiesel by Response Surface Methodology. Bioresour Technol 88: 103-106.
- Chen CS, Liu KJ, Lou YH, Shieh CJ (2002) Optimisation of Kojic Acid Monolaurate Synthesis PS from Pseudomonas cepacia, J Sci Food Agric 82: 601-605.
- Soo EL, Salleh AB, Basri M, Rahman RNZA, Kamaruddin K (2004) Response Surface Methodology Study on Lipase-catalyzed Synthesis of Amino Acid Surfactants. Process Biochem 39: 1511-1518.
- Gunawan ER, Basri M, Abd Rahman MB, Salleh AB, Abd Rahman RNZ (2005) Study on Response Surface Methodology (RSM) of Lipase-catalyzed Synthesis of Palm-based Wax Esters. Enzyme Microb Technol 37: 739-744.
- Jei FS, Wu HZ, Shieh CJ (2003) Optimised of Enzymatic Synthesis of Propylene Glycol Monolaurate by Direct Esterification. Food Chem 8: 91-96.
- Bidin H, Basri M, Mat Radzi S, Ariff A, Abdul Rahman RNZ, Salleh AB (2009) Optimization of Lipase-catalysed Synthesis of Palm Amino Acid Surfactant using Response Surface Methodology (RSM). Ind Crops Prod 30: 206-211.
- Lee, Chaibakhsh N, Abdul Rahman MB, Basri M, Tejo BA (2010) Optimized Enzymatic Synthesis of Levulinate Ester in Solvent-free System. Ind Crops Prod 10: 1016.
- Melo LLMM, Pastore, GM, Macedo GA (2005) Optimized Synthesis of Citronella Flavor Esters using Free and Immobilized Lipase from Rhizopus sp. Process Biochem 40: 3181-3185.
- Chaabouni MK, Ghamgui H, Bexxine S, Rekik A, Gargouri Y (2006) Production of Flavor Esters by Immobilized Staphylococcus simulans Lipase in a Solvent Free System. Process Biochem 41: 1692-1698.
- Keng PS, Basri M, Abd Rahman MB, Salleh AB, Abd Rahman RNZ, Ariff A (2005) Optimization of Palm-based Wax Esters Production using Statistical Experimental Designs. J Oleo Sci 54: 519-528.
- Salah RB, Ghamghui H, Miled N, Mejdoud H, Gaigouri Y (2007) Production of Butyl Acetate Ester by Lipase from Novel Strain of Rhizophus oryzae. J Biosci Bioeng 4: 368-372
- Hari krishna S, Sattur AP, Karanth AP (2001) Lipase Catalysed Synthesis of Isoamyl Isobutyrate - Optimisation using Central Composite RotaTable Design. Process Biochem 37: 131-136.
- Mat Radzi S, Basri M, Salleh AB, Ariff A, Mohamad R, Abdul Rahman MB, Raja Abdul Rahman RNZ (2006) Optimization Study of Large Scale Enzymatic Synthesis of Liquid Wax Ester by Response Surface Methodology. J Chem Technol. Biotechnol 81: 374-380.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 14213
- [From(publication date):
June-2011 - Nov 24, 2024] - Breakdown by view type
- HTML page views : 9725
- PDF downloads : 4488