Research Article Open Access
Microbial degradation of Textile Dye (Remazol Black B) by Bacillus spp. ETL-2012
| Maulin P Shah*, Patel KA, Nair SS and Darji AM | |
| Applied and Environmental Microbiology Lab, Enviro Technology Limited (CETP), Ankleshwar- 393 002, Gujarat, India | |
| Corresponding Author : | Maulin P Shah Applied and Environmental Microbiology Lab Enviro Technology Limited (CETP) Ankleshwar- 393 002 Gujarat, India Tel: +91-90 999 65504 Fax: +91-2646-250707 E-mail: shahmp@uniphos.com |
| Received December 25, 2012; Accepted January 28, 2013; Published January 30, 2013 | |
| Citation:Shah MP, Patel KA, Nair SS, Darji AM (2013) Microbial degradation of Textile Dye (Remazol Black B) by Bacillus spp. ETL-2012. J Bioremed Biodeg 4:180. doi: 10.4172/2155-6199.1000180 | |
| Copyright: © 2013 Shah MP, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
In the present study, an attempt was made to examine the potential of isolated bacterium for decolorization of
Remazol (azo dye) in batch reactors. Bacteria from soil, and dye waste were subjected to acclimatization with azo dye, in the basal nutrient media. The most promising bacterial isolate was used for further dye degradation studies. Biochemical characteristics revealed the isolated organism as Bacillus spp. The optimum pH and temperature for the decolourization was 6.0 and 37°C, respectively. This decolorization potential increased the applicability of this microorganism for the dye removal. The results suggest that the Bacillus spp. ETL-2012 can be used as a useful tool to treat waste water containing reactive dyes.
| Keywords |
| Remazol Black B; Bacillus; pH; Temperature; Static condition |
| Introduction |
| Rapid industrialization has necessitated the manufacture and use of different chemicals in day to day life [1]. The textile industry is one of them, which extensively use synthetic chemicals as dyes. Wastewaters from textile industries pose a threat to the environment, as large amount of chemically different dyes are used. A significant proportion of these dyes enter the environment via wastewater. Approximately 10,000 different dyes and pigments are used industrially and over 0.7 million tons of synthetic dyes are produced annually, worldwide [2]. Pollution due to textile industry effluent has increased during recent years. Moreover, it is very difficult to treat textile industry effluents, because of their high Biological Oxidation Demand (BOD), Chemical Oxygen Demand (COD), heat, color, pH and the presence of metal ions [3]. The textile finishing generates a large amount of waste water containing dyes and represents one of the largest causes of water pollution [4], as 10-15% of dyes are lost in the effluent during the dyeing process [5]. The traditional textile finishing industry consumes about 100 liters of water to process about 1 Kg of textile material. The new closed-loop technologies such as the reuse of microbial or enzymatical treatment of dyeing effluents could help reducing this enormous water pollution [6]. Azo dyes have been used increasingly in industries because of their ease and cost effectiveness in synthesis compared to natural dyes. However, most azo dyes are toxic, carcinogenic and mutagenic [7]. Azo bonds present in these compounds are resistant to breakdown, with the potential for the persistence and accumulation in the environment [8]. However, they can be degraded by bacteria under aerobic and anaerobic conditions [9]. Several physico-chemical techniques have been proposed for treatment of colored textile effluents. These include adsorption on different materials, oxidation and precipitation by Fenton’s reagent, bleaching with chloride or ozone photo degradation or membrane filtration [10]. All these physical or chemical methods are very expensive and result in the production of large amounts of sludge, which creates the secondary level of land pollution. Therefore, economic and safe removal of the polluting dyes is still an important issue. Bioremediation through microorganisms has been identified as a cost effective and environment friendly alternative for disposal of textile effluent [11,12]. In recent years a number of studies have focused on some microorganisms capable of degrading and absorbing dyes from wastewater. A wide variety of microorganisms are reported to be capable of decolonization of dyes [11-24]. The current study has evaluated the potential of isolated bacterial strain from textile effluent for their decolorization efficiency of the textile dye, Remazol Black B under in vitro conditions and optimization of the factors influencing the process. |
| Materials and Methods |
| Sampling and analysis of effluent |
| The effluent sample was collected from the middle point of the area. Standard procedures (Spot and Grab) were followed during sampling. The temperature and pH were determined at the sampling site. The pH was determined by using pH meter and temperature with laboratory thermometer. The sample was transported to laboratory at 4°C as in accordance with the standard methods [25]. The physicochemical parameters such as colour, Biological Oxidation Demand (BOD), Chemical Oxygen Demand (COD), Total Suspended Solids (TSS), and Total Dissolved Solids (TDS) were determined as soon as the sample was brought to the laboratory. Sample color was analyzed by spectrophotometer (SHIMADZU UV-1800). BOD was determined by employing evaporation method by DO meter (WTW Germany) while COD was measured by COD instrument directly (Spectralab, India). |
| Microorganism and culture conditions: The method included two steps: Treatability test was carried out by enrichment culture technique and toxicity test in which, toxicity was assessed in a series of parallel flasks with geometrically increasing concentration of Remazol Black B. Microbial growth was measured daily by turbidity. The concentration at which the toxic components inhibited growth was noted and was used as a warning of an upper concentration limit. Isolation was done on nutrient agar plates incorporated with 100 mg/l Remazol Black B. Pure culture was maintained on the nutrient agar slants. |
| Chemicals: The textile dye, Remazol Black B (λmax 595 nm) was obtained from local textile industries, Ankleshwar, Gujarat, India. A stock solution of the dye (1000 mg L–1) was prepared in de-ionized water and used for all studies. |
| Isolation, screening and identification of dye decolorizing bacteria from effluent: The textile effluent was collected in sterile collection tubes from the sludge and wastewater of the ditches at industrial site located in Ankleshwar Textile Industries, Gujarat, India. The sample collected from the textile mill was screened for azo dye decolorizing bacterial strains by inoculating 10 ml of sludge solution into 250 ml Erlenmeyer flask containing 100 ml nutrient broth (g/L as mentioned above). The flasks were incubated at 37°C under shaking conditions (120 rpm). After 48 h of incubation, 1.0 ml of the culture broth was appropriately diluted and plated on Nutrient Agar containing 100 mg L–1 Remazol Black B. The Morphologically distinct bacterial isolates showing clear zones around their colonies due to decolorization of dye were selected for further studies. The pure culture stocks of these isolates were stored at 4°C on Nutrient Agar slopes containing 1000 mg L–1 of Remazol Black B. These isolates were screened for their ability to decolorize Remazol Black B in liquid culture. The Screening process in liquid media was carried out by inoculating a loop full of cultures exhibiting clear zones into Nutrient broth containing Remazol Black B under static conditions. After 24 h of incubation, 1ml. of cell suspension was transferred to fresh nutrient broth containing Remazol Black B to screen the strains with color removing ability. The Screening procedure in liquid medium was continued until complete decolorization of broth. A small amount of decolorized broth was transferred to nutrient agar plates containing Remazol Black B (100 mg L–1). The bacterial isolate which tolerated higher concentration of the azo dye was isolated by streak plate method. The azo dye decolorizing bacteria was identified from several aspects including morphology characters, biochemical tests as described in Bergey’s manual of determinative bacteriology (Indole, Methyl Red, Voges-Proskauer test, Citrate, Catalase, Oxidase, Nitrate Reduction test, Hydrolysis of Casein, Starch, Urea and Gelatin). Assimilation of various sugars such as D-glucose, D-fructose, galactose, mannitol and D-maltose as sole carbon source was determined by inoculating the isolate into carbohydrate broth supplemented with respective carbon source. After inoculation the tubes were incubated at 37°C for 24-48 h. |
| Analyses of 16S rRNA sequences |
| Genomic DNA of the isolate was extracted with a GenElute DNA extraction kit from Sigma. The 16S rRNA gene of isolate was amplified using the universal primers 8F (5′-AGAGTTTGATCCTGGCTCAG) and 1541R (50-AAGGAGGTGATCCAGCCGCA-3′). The amplification was done by initial denaturation at 95°C for 5 min followed by 10 cycles of 93°C for 1 min, 63°C for 1 min, 71°C for 1.5 min; 20 cycles of 93°C for 1 min, 67°C for 1 min, 71°C for 2 min and final extension at 71°C for 5 min. The purified PCR product was sequenced in both directions using an automated sequencer by Perkin Elmer ABI Prism 377 DNA sequencer. The phylogenic relationship of the isolate was determined by comparing the sequencing data with sequences of some members of the genus Bacillus available through the GenBank database of the National Center for Biotechnology Information. The gene sequences of each isolate obtained in this study were compared with known 16s rRNA gene sequences in the GenBank database. |
| Decolorization experiments |
| Bacillus spp. ETL-2012 was grown for 24 h at 37°C on nutrient agar. 10% inoculum (OD600 1.0) the isolate was inoculated in nutrient broth to study the decolorizing ability of the culture. The dye was filter sterilized by using 0.2 μm filter (Sartorius Biolab, Germany) and added after sterilization of medium throughout the study. The dye (100 mg/l) was added immediately and incubated under static condition at 37°C. Aliquot (3 mL) of culture media was withdrawn at different time intervals and centrifuged at 8,000 g for 15 min. Decolorization was monitored by measuring the absorbance of the culture at λmax of the dye i.e., 595 nm and change in pH was also recorded. Biomass in the dye containing medium was determined by wet weight method. |
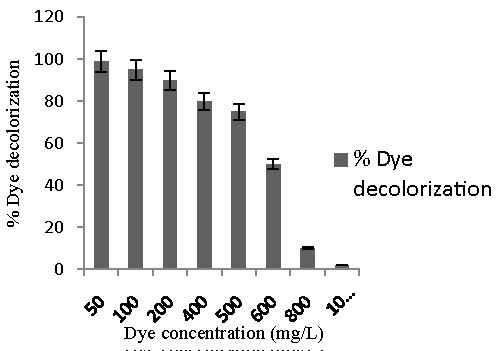
| Decolorization at different dye concentration: In order to examine the effect of initial dye concentration on decolorization under static condition 50–1000 mg/l of Remazol Black was added to the sterile nutrient broth inoculated with 10% Bacillus spp. ETL-2012 of (OD600 1.0) and incubated at 37°C under static condition. The % decolorization was measured. All decolorization experiments were performed in three sets. Abiotic controls (without culture) were always included. The %decolorization and average decolorization rate was measured. |
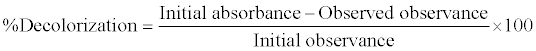 (1) (1) |
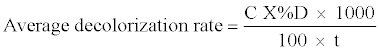 (2) (2) |
| Where C = initial concentration of dye mg/l, %D = dye decolorization % after time t [26]. |
| Effect of pH on dye decolorization: Sterile nutrient broth of different pH 3, 4, 5, 6, 7, 7.5 and 8 was inoculated with 10% inoculum and incubated at 37°C under static condition. The dye concentration was 100 mg/l. All decolorization experiments were performed in three sets. Abiotic controls were also set. The %decolorization was measured as mentioned earlier. |
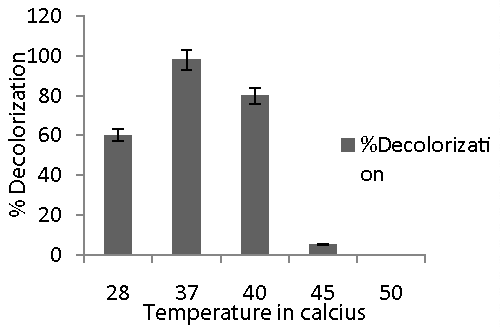
| Effect of temperature on dye decolorization: Sterile nutrient broth of pH 7.5 was inoculated with 10% inoculum and filter sterilized dye at 100 mg/l was added after sterilization. The broth was incubated at 28, 37, 40, 45 and 50°C. The experiment was carried out in triplicate. Abiotic controls (without microorganism) were always included. The %decolorization was measured. |
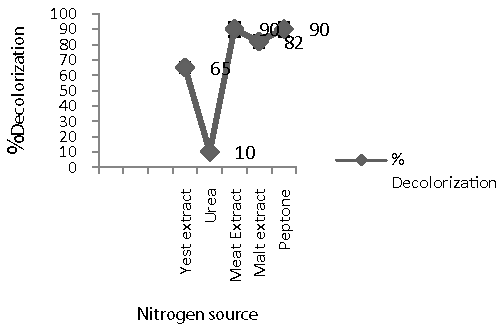
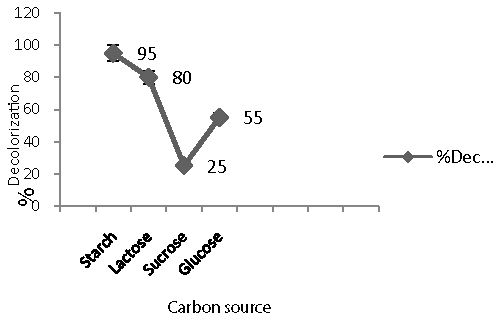
| Effect of carbon and nitrogen sources on decolorization: To study the effect of carbon and nitrogen sources on decolorization of Remazol Black B, semi synthetic medium was used [26]. It was further incorporated with different carbon and nitrogen sources (1% each) such as glucose, sucrose, lactose and starch, yeast extract, peptone, malt extract, meat extract and urea, respectively [26]. 100 mg/l of the dye was used. Filter sterilized dye was added after sterilization of the medium and after inoculation of the isolate. |
| Repeated dye decolorization in fed batch process: Repetitive decolorizing ability of the cells was (repeated use) studied by addition of the dye (100 mg/l) at each cycle [27]. |
| Results and Discussion |
| Physico-chemical characterization of textile effluent |
| Industrial effluent is not stable and it varies often in a wide range depending upon the process practiced. South Asian countries are experiencing severe environmental problems due to rapid industrialization. This phenomenon is very common where the polluting industries like textile dyeing, leather tanning, paper and pulp processing, sugar manufacturing, etc. thrive as clusters. Among these the textile industries are large industrial consumers of waters as well as producers of wastewater. The effluent discharged by this industry leads to serious pollution of groundwater and soils and ultimately affects the livelihood of the poor [28]. The physico-chemical characterization of the collected textile effluent sample from Ankleshwar textile industries, Ankleshwar, Gujarat, India, showed a high load of pollution indicators. Colour is contributed to a water body by the dissolved compounds (dyes and pigments). The effluent color was black due to mixture of various dyes and chemicals used in the dyeing process. The pH of tested sample was slightly alkaline when compared to the acidic pH of the dyeing effluent in a previous study [29]. The pH of the effluent alters the physico-chemical properties of water which in turn adversely affects aquatic life, plants and humans. The soil permeability gets affected resulting in polluting underground resources of water. The temperature of the effluent was high in comparison with the temperature of another effluent in one study [30]. High temperature decreases the solubility of gases in water which is ultimately expressed as high BOD/COD. The values of BOD and COD were within the permissible limits in the present sample in comparison to the very high values of BOD and COD in one effluent study. Sediments rate is drastically increased because of high value of TDS which reduces the light penetration into water and ultimately decrease the photosynthesis. The decrease in photosynthetic rate reduces the DO level of wastewater which results in decreased purification of wastewater by microorganisms [31]. The current sample exhibited high values of heavy metals which were of the same order of magnitude reported in another effluent sample [32]. The nutrients of the surrounding soils are depleted as a result of high value of heavy metals thereby affecting soil fertility. High chloride contents are harmful for agricultural crops if such wastes containing high chlorides are used for irrigation purposes [33]. Majority of the textile effluent samples have permissible limits of sulphate ions. The effluent showed phenolic contents greater than 0.1 ppm which is though permissible limit of the phenolic compounds still these compounds are very toxic to fish even at very low concentrations [34]. The bleaching and dying process are the main causes of pollutants which include caustic soda, hypochlorite and peroxides. The isolation of different microorganisms from the effluent sample collected from the Ankleshwar Textile Industries, Gujarat, India, indicates to natural adaptation of microorganisms to survive in the presence of toxic chemicals and dyes. Interest in the bioremediation of pollutants using bacteria has intensified in recent years, as many researches demonstrated the efficacy of bacterial bioremediation over fungal and actinomycetes. Many bacteria capable of reducing azo dyes reported were isolated from textile effluent contaminated sites [35]. The effluent sample collected from Ankleshwar textile industries, Ankleshwar, Gujarat, India, was black in color, with pungent smell and pH of slightly above neutral level and was within the permissible limits. The temperature of the effluent was high. TSS and TDS in the textile effluent were very high. The solids present in ground water, besides effecting the growth of the plants directly, also affect the soil structure, permeability and aeration, indirectly effecting the plant growth. The BOD values were within the permissible limits in the effluent sample. Different bacterial strains isolated from the textile effluent were screened for their ability to decolorize the textile azo dye and the potential strains were characterized morphologically and biochemically for identification. |
| Isolation and identification |
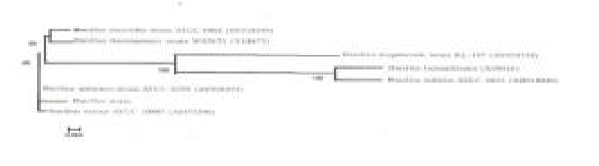
| The study was started by screening for potential textile azo dye decolorizing bacteria isolated from the textile industry effluent. Colonies surrounded by a nearly decolorized zone were isolated and then tested for dye removal capability using submerged culture. Strains isolated from the white colonies were inoculated in 100 ml of Nutrient broth in a 250 ml conical flask and incubated at 37°C under static conditions. One strain exhibiting highest decolorizing activity was chosen for further studies. According to The Bergey’s manual of systematic bacteriology and considering the physiological and biochemical tests performed, the strain was tentatively named as Bacillus sp. strain ETL-2012. To confirm the identity of the isolate, PCR amplification and sequencing of the 16S rRNA gene were done. Figure 1 shows phylogenetic relationships derived from 16S rRNA gene sequence analysis of strain ETL-2012 with respect to Bacillus species. The tree was constructed using the neighbour-joining [36]. Among the described sub species, the closest relative of isolate ETL-2012 was Bacillus cereus. |
| Effect of pH |
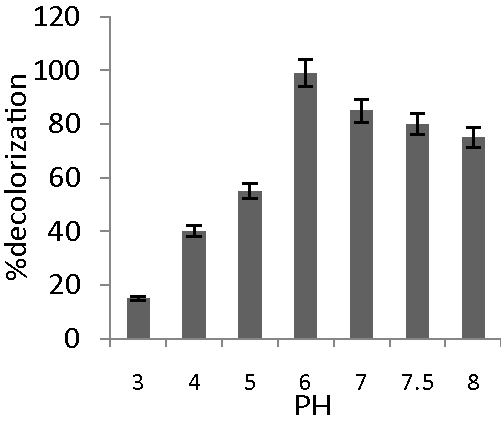
| Bacterial cultures generally exhibit maximum decolorization at pH values near 7.0. Our culture exhibited decolorization activity in the range of pH 5–8. At pH 3 and 4 decolorization was 15 and 40% respectively. The isolate showed more or less constant decolorization between pH 5–8. Maximum decolorization (99.00%) was at pH 6 (Figure 2). Bacillus spp. ETL-2012 grows very well in the pH range of 5–9. E. coli and Pseudomonas luteola both exhibited best decolorization at pH 7.0 with constant decolorization rate up to pH 9.5 [14]. |
| Effect of temperature |
| The rate of chemical reaction is the direct function of temperature. Bacteria require optimum temperature for growth. Since dye decolorization is metabolic process hence shift in temperature from optimum results into decrease in dye decolorization as high temperature causes thermal inactivation of proteins and possibly of such cell structures such as membrane. The isolate showed complete decolorization at 27, 37 and 40°C but rapid decolorization i.e., within 12 h was observed at 37°C (Figure 3). This could be due to a greater production of enzymes and optimal growth conditions of the isolate for its dye decolorizing ability. The decolorization at this optimum temperature may be owing to higher respiration and substrate metabolism. This also demonstrates that decolorization of the dye was through microbial reaction which relies on optimal temperature and not by adsorption. There was no decolorization at 45 and 50°C. Hence pH and temperature optima for Remazol Black B were found to be pH 6 and 37°C respectively. |
| Effect of initial dye concentration |
| Figure 4 showed decolorizing ability of our culture increased with increase in dye concentration from 50 to 500 mg/l. The activity was lower at dye concentration 600 mg/l and above which decolorization was strongly inhibited at dye concentration 1000 mg/l (Figure 4). It has been proposed that dye concentration can influence the efficiency of microbial decolorization through a combination of factors including the toxicity imposed by dye at higher concentration. Thus, the isolate which could decolorize dye much above the reported dye concentration in wastewater, can be successfully employed for treatment of dye bearing industrial wastewater. |
| Effect of carbon and nitrogen sources on decolorization |
| While trying to enhance decolorization performance of Remazol Black B, extra carbon and nitrogen sources was supplied. Percentage decolorization (95%) (Figure 5) was maximum with purified substrate peptone while less decolorization with other supplements of carbon and nitrogen source. Similar result was also reported by for decolorization of C.I.Reactive Green 19A. [26]. The culture showed negligible decolorization in the presence of sucrose (25%) whereas moderate activity was shown in presence of glucose (55%), lactose (80%) and maximum decolorization was reported in presence of starch (95%) (Figure 6). In addition, supplying urea as a nitrogen source exhibited |
| less decolorizing ability. Similar results were reported by Saratale [26]. In contrast, addition of carbon sources seemed to be less effective to promote the decolorization probably due to the preference of the cells in assimilating the added carbon sources over using the dye compound as the carbon source [26]. Decolorization with repeated addition of dye aliquots. The repeated use of microorganisms is important in commercial point of view. This study was carried out to examine the ability of Bacillus spp. ETL-2012 to decolorize repeated addition of Remazol Black B dye aliquot (100 mg/l) at static condition. The isolate had an ability to decolorize 100% dye up to third dye aliquot addition and after that subsequent cycle required 10-12 h. The eventual cessation of decolorization was likely due to nutrient depletion [26,27]. Thus Bacillus spp. ETL-2012 showed the ability to decolorize repeated addition of dye aliquots which is noteworthy for its commercial applications |
| Conclusion |
| Although decolorization is a challenging process to both the textile industry and the waste water treatment, the result of this findings and literature suggest a great potential for bacteria to be used to remove color from dye wastewaters. Interestingly, the bacterial species used in carrying out the decolorization of azo dye Remazol Black B in this study was isolated from the textile dye industry waste effluent. The bacterial strain Bacillus spp. ETL-2012 showed decolorizing activity through a degradation mechanism. This observation has established that the bacteria are adaptive in nature and can degrade contaminants. The ability of the strain to tolerate, decolorize azo dyes at high concentration gives it an advantage for treatment of textile industry waste waters. However, potential of the strain needs to be demonstrated for its application in treatment of real dye bearing waste waters using appropriate bioreactors. |
References
|
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
 |
 |
 |
| Figure 4 | Figure 5 | Figure 6 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 16245
- [From(publication date):
February-2013 - Nov 24, 2024] - Breakdown by view type
- HTML page views : 11302
- PDF downloads : 4943
