Research Article Open Access
Biodegradation of Phenolic and Polycyclic Aromatic Compounds by Some Algae and Cyanobacteria
| Mostafa M El-Sheekh1*, MM Ghareib2 and GW Abou-EL-Souod2 | |
| 1Botany Department, Faculty of Science, Tanta University, Tanta, Egypt | |
| 2Botany Department, Faculty of Science, Menoufia University, Egypt | |
| Corresponding Author : | Mostafa M. El-Sheekh Botany Department, Faculty of Science Tanta University, Tanta, Egypt Fax: 20-40-3350804 E-mail: mostafaelsheekh@yahoo.com |
| Received November 07, 2011; Accepted December 06, 2011; Published December 08, 2011 | |
| Citation: EI-Sheekh MM, Ghareib MM, EL-Souod GW A (2011) IBiodegradation of Phenolic and Polycyclic Aromatic Compounds by Some Algae and Cyanobacteria. J Bioremed Biodegrad 2:133. doi: 10.4172/2155-6199.1000133 | |
| Copyright: © 2011 EI-Sheekh MM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
In this work, the oxidation of phenolic compounds was accompanied by shift in the wavelength and change in the colour such as the oxidation of phenol to catechol by Volvox aureus, Nostoc Linckia and Oscillatoria rubescens . The oxidation of β-naphthol by Volvox aureus, Lyngbya lagerlerimi and Nostoc linckia , and the oxidation of catechol by Chlorella vulgaris and V. aureus were suggested. The degradation of polycyclic aromatic hydrocarbons by different algae seems to be related to the molecular structures of the compound and physiological metabolism of the algae. The highest percentage of degradation of naphthalene by N. linckia after 7 days was 47.71%, while the highest percentage of degradation of anthracene by E. viridis after 7 days was 92.28%. The highest percentage of degradation of 2-methythie 3-phenyl quinazlin-4- 3H (one) by V. aureus after 7 days was 83.39%, and the highest percentage of degradation of 2- phenyl 3,1benzexazin-4 one by E. viridis after 7 days was 79.74%. The obtained results suggest that microbial biodegradation of pollutants can be used to clean up contaminated environments. Biotreatment of wastes using living organisms is an environmentally friendly, relatively simple and cost-effective alternative to physico-chemical processes.
| Keywords |
| Algae; Biodegradation; Phenols; Polycyclic aromatic compounds |
| Introduction |
| Some algae that exist in polluted water are being used as indicators of pollution, and some of the selective types of algae make or play the role in the degradation of industrial pollutants. Cyanobacteria (blue green algae) and eukaryotic micro algae were capable of biotransforming naphthalene to more water soluble phenol, 1-naphthol [1]. Scendesmus obliques is able to utilize naphthalene sulphonic acids as a sulfur source for their biomass with releasing the carbon ring into the medium. The algae could use nitro and amino- substituents, from amino naphthalenes, and amino- and nitrobenzoates as nitrogen sources, and chlorobenzoates could be dehalogenated and the chloride being accumulated by the cells [2]. Phenol removal levels of Synechocystis sp. was investigated in BG11 medium with 10 mg/L triacontanol (TRIA) and without it to test whether the hormone could increase the removal efficiency by increasing biomass [3]. |
| Metabolism of phenolics by Ochromonas danica was obligatorily aerobic. There was no phenol turn over under anaerobic condition (O2- free nitrogen) until air was admitted when phenol removal commenced [4]. There are few examples of algae degrading aromatic compounds [5,6] examined the effects of the chlorophyte alga, Selenstrum capriconutum, on benzo pyrene. They found that algae used a dioxygenase system to oxidize the compound to cis dihydrodiols which were then converted to sulfate ester and glucoside conjugates. |
| The anaerobic pathway for breakdown of the aromatic ring was different and quite distinct from the aerobic pathway [7]. Anaerobic biodegradation of 11 simple aromatic lignin derivatives to methane was investigated, suggesting that more half of the carbon associated with the 11 aromatic compounds could be potentially converted to methane gas [8]. Benzene undergoes an initial ring reduction followed by hydrolytic ring cleavage to yield aliphatic acids for cell growth. Microorganisms, however, can utilize a remarkable biochemical pathway of initial ring reduction followed by ring disruption to initiate biotransformation of aromatic hydrocarbons. Molecular oxygen is necessary for aromatic catabolism as it is directly incorporated into the aromatic ring structure [9]. |
| Algae can convert benzo pyrene to peroxides, dihdrodiols and oxides [10,11]. When benzo pyrene was incubated with algae, a greater percentage of the benzo pyrene was first degraded and mineralized (broken down to carbon dioxide and water). These findings that algae in conjunction with bacteria may lead to more complete degradation of large PAHs like benzo pyrene [12]. |
| Recently, [13-16] studied the ability of some algae for biodegradation of phenolic compounds in the light and dark. The aim of this investigation was to study the ability of some algae isolated from different polluted sites, on the degradation of different phenolic compounds, polycyclic and heterocyclic aromatic compounds. |
| Materials and Methods |
| Algae and growth conditions |
| The following algae are isolated from different polluted sites and purified in axenic cultures (bacterial free) and used in this investigation [17]. The green algae are Chlorella vulgaris, Elkatothrix viridis and Volvox aureus and the blue green algae are Lyngbya lagerlerimi, Nostoc linckia, Oscillatoria rubescens. The green algae were cultured in flasks containing a sterile Bold’s Basal medium [18]. Inoculated flasks were kept in a culture room at a temperature of 25 ±1Ã?Â?C (pH 7.0) under continuous light with intensity of 5000 lux. Cyanobacteria were maintained on Allen medium [19] with the same previous conditions except the light intensity was 3000 lux. |
| Degradation of the pollutants by the isolated algae |
| Preliminary experimentations were carried out to show the interaction of the tested pollutants with the media. In 120 ml of the sterile medium was previously introduced into clean sterilized 250 ml Erlenmeyer flask. The following pollutants were added, a- naphthol (25 mg.L-1), b-napthol (25 mg.L-1), phenol (166 mg.L-1), catechol (60 mg.L-1), anthracene (60 mg.L-1), Naphthalene (40 mg.L-1), 2 methyl thie- 3 phenyl quinazlin – 4 (3H) one add (20 mg.L-1), and 2 phenyl 3,1 benzexazin – 4 (one) 20 mg.L-1. The proper experimental period was found to be seven days, to observe the change in the peak. Stock cultures of each organism were prepared by inoculating 90 ml of the sterile medium in sterilized 250 ml Erlenmeyer flasks. (103 cells / ml) of algae were introduced separately into the flasks containing the pollutants with different concentration. The tested pollutants were added to the algal cultures to acclimate the algae. Inoculated flasks were kept in a culture room at temperature of 25 +1Ã?Â?C pH (7.5) and with a daily photoperiod of 16 hr light (5000 +200 Lux) and 8 hr darkness for green algae and 300 Lux for cyanobacteria. |
| Spectroscopic analysis |
| The culture was centrifuged at 5000 rpm for 15min. The supernatant was evaluated via light absorption method and percentage reduction rates were calculated. Some of the suspension was assayed with the (UV-Visible-Unvisible spectrum method) using [U.V] Perkin-Elmer Lambda 4B. Accessory interface U.V vis. Spectrophotometer. |
| Infrared measurements |
| Infrared analysis was introduced to identify the structural variation using [Perkin-Elmer] infrared data station [1430 Ratio-Recording Infrared spectrophotometer]. The infrared analysis for the biomass of algae before and after treatment of chemical compounds was done to judge weather the cells sorb some of these compounds. The infrared analysis was also done to the supernatant to observe the change of the intensity peak of the compound and hence the ability of the algae to degrade these compounds. |
| Results |
| Degradation of different Phenolic compounds by Lyngbya lagerlerimi |
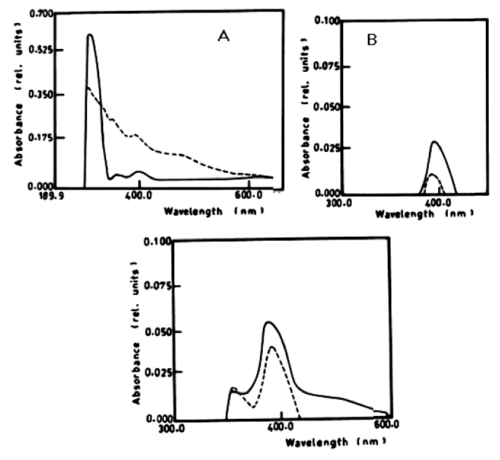
| According to table 1 and figure 1A-1C the degradation of anaphthol by Lyngbya lagerlerimi at wavelength 301nm was 36.6% after 5days of incubation. The oxidation of b-naphthol by L. lagerlerimi at wavelength 322nm is accompanied by shift in wavelength at 387nm, high absorbance and change in colour. The percentage degradation of phenol by L. lagerlerimi was 64.4% after 3 days. The two peaks at wavelengthes 268 and 203 nm were disappeared while one peak was appeared at wavelength 388 nm. The degradation of catechol by L. lagerlerimi was 23.8% after 5 days of incubation. |
| Degradation of different phenolic compounds by Nostoc linckia |
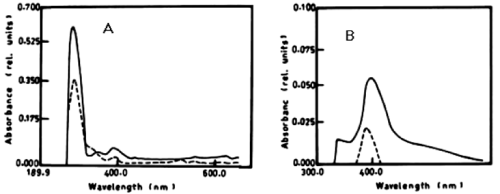
| The degradation of a- naphthol by Nostoc linckia at wavelength 301nm after 3 days was 40.56% and this ratio was constant during the incubation period. The oxidation of phenol by N. linckia is accompanied by shift in the wavelength at 386 nm which represent the wavelength of catechol and this accompanied by change in colour. The catechol was degraded by N. linckia at wavelength 386 nm, and the percentage of degradation after 3 days was 56.36%, and after 5 days it was almost constant being 56.38%, as compared with the control as shown in table 1 and (Figure 2A, 2B). |
| Degradation of different phenolic compounds by Oscillatoria rubescens |
| The results presented in table 1 and show the degradation of a-naphthol by at wavelength 301 nm. The degradation ratio was 44.41% - 59.5%, during the incubation period. table 1 also shows that b-naphthol was degraded by O. rubescens at wavelength 322 nm after 7 days and the percentage of degradation was 3.04%. The oxidation of phenol by O. rubescens is accompanied by shift in the wavelength at 386 nm which fit to the wavelength of catechol, and this also accompanied by change in colour and high absorbance. Catechol was degraded by O. rubescens at wavelength 386 nm, and the percentage of degradation after 7 days was 2.11%. |
| Degradation of different phenolic compounds by Chlorella vulgaris |
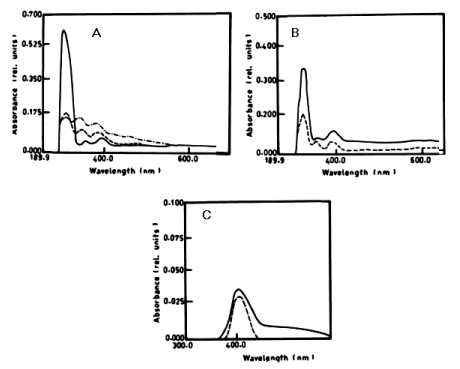
| The percentage of a-naphthol degradation by Chlorella vulgaris at wavelength 301 nm after 3 days was 68.68%, 5 days was 71.19% and after 7 days it was 71.20%. The degradation of b-naphthol by C. vulgaris at wavelength 322 nm was 52.80%, during 7 days of incubation. The percentage of degradation of phenol by C. vulgaris at wavelength 388 nm after 7 days was 9.05% as shown in table 1 and (Figure 4A-4C). |
| Degradation of different phenolic compounds by Elkatothrix viridis |
| According to table 1 and figure 5A-5C the degradation of a-naphthol by Elkatothrix viridis at wavelength 301 nm was 6.47%, 8.10% after 3 and 7days of incubation, respectively. The percentage degradation of b-naphthol by E. viridis was 6.11%, after 7 days of incubation. The percentage degradation of phenol by E. viridis was 5.05 % after 7 days. The degradation of catechol by E. viridis at wavelength 386 nm, after 3 days was 52.50%, and this ratio was constant after 5 and 7 days of incubation. |
| Degradation of different phenolic compounds by Volvox aureus |
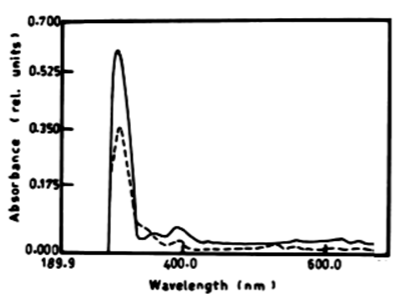
| The results presented in table 1 and figure 6A, 6B show the degradation of a-naphthol by V. aureus at wavelength 301 nm. The percentage of degradation after 3 days was 2.11%, and this ratio remained constant till the end of incubation period and was accompanied by shift in the wavelength. The oxidation of b-naphthol by V. aureus at wavelength 301 nm was accompanied by shift in the wavelength at 387 nm, and this is accompanied by high absorbance and change in colour. The oxidation of phenol by V. aureus at wavelength 388 nm after 3 and 5 days was accompanied by shift in the wavelength at 386 nm which represent the wavelength of catechol, and this is accompanied by high absorbance and change in colour. The oxidation of catechol by V. aureus at wavelength 386 nm after 3 and 5 days was also shown by shift in the wavelength at 391 nm, and this is accompanied by high absorbance and change in colour. |
| Degradation of polycyclic aromatic hydrocarbons by different algae |
| According to Table 2 the degradation of naphthalene by Lyngbya lagerlerimi at wavelength 388 nm after 3 days of incubation was 14.94% and 22.69% after 7 days. The percentages of degradation of anthracene by L. lagerlerimi were 22.71%, 26.27%, and 26.29%. There was no significant degradation of naphthalene evident by N. linckia at wavelength 388 nm, while as the percentage of degradation of anthracene was slightly detected after 7 days of incubation. O. rubescens, at wavelength 388 nm also showed as low percentage of degradation as 2.13% after 7 days of incubation. The degradation of anthracene by O. rubescens at wavelength 392 nm were 57.43%, 76.45% and 76.47% after 3, 5 and 7 days of incubation respectively (Table 2) (Figure 7). |
| Degradation of heterocyclic aromatic compounds by different algae |
| Table 2 shows the degradation of 2-methyl thie-3-phenyl quinazlin 4-(3H) one by L. lagerlerimi at wavelength 260 nm. The percentage of degradation after 3, 5, 7 days of algal growth were 67.29%, 77.10% and 77.11% respectively. The percentage of degradation of 2-phenyl 3, 1 benzexian-4 one by L. lagerlerimi were 22.13%, 33.39% and 33.40% after 3, 5 and 7 days of incubation, respectively. The degradation of 2-methyl thie-3 phenyl quinazlin- 4 (3H) one by N. linckia at wavelength 260 nm were 27.56% and 54.20% after 3, 5 days respectively. The percentages of degradation of 2-phenyl-3,1 benzexian- 4 one by N. linckia were 35.3%, 46.46% and 46.47% after 3, 5 and 7 days of incubation, respectively. |
| The degradation of 2-methyl thie-3 phenyl quinazlin-4 (3H) one by O. rubescens at wavelength 260 nm. The percentage of degradation seemed not changed over the incubation period. However, the percentage of degradation of 2-phenyl-3,1 benzexian- 4 one by O. rubescens after 3 days was 53.24%, increased to 71.42% after 5 days (Table 2). |
| Degradation of polycyclic aromatic hydrocarbons by different algae |
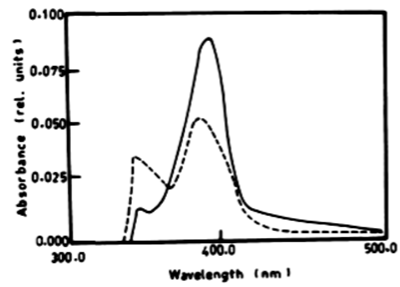
| From the results in Table 2 and figure 8A-8D it is evident that the percentage of degradation of anthracene by C. vulgris at wavelength 392 nm was 75.47%, 83.30% and 83.37% after 3, 5 and 7 days respectively. The degradation of naphthalene by C. vulgris at wavelength 388 nm after 7 days was 28.62%. The degradation of anthracene by E. viridis at wavelength 392 nm after 3 was 57.66% and 92.28% after 7 days of incubation. The degradation of naphthalene by E. viridis at wavelength 388 nm after 3, 5 and 7 days was more or less the same and its percentage was 12.89%. Table 2 and Figure 9 show that the degradation of anthracene by V. aureus at wavelength 392 nm was 40.82% after 7 days of incubation. However, the percentage of degradation of naphthalene by V. aureus was 3.04% after 7 days of incubation. |
| Degradation of heterocyclic aromatic compounds by different algae |
| The percentages of degradation of 2-methyl thie 3 phenyl quinazlin 4-(3H) one by Chlorella vulgaris at wavelength 260 nm were 38.52%, and 40.51% after 3, and 7 days respectively. There was no significant changes in the degradation of 2-phenyl 3,1 benzexazin 4-one evident by C. vulgris at wavelength 2e84 nm. The degradation of 2-methyl thie 3 phenyl quinazlin 4-(3H) one at wavelength 260 nm by E. viridis was 67.24% and 71.23% after 5 and 7 days respectively. The percentage of degradation of 2-phenyl 3,1 benzexazin 4-one by E. viridis at wavelength 284 nm after 3 days was 67.20%, and after 5 days of incubation was 79.73%. The degradation of 2-methyl thie 3 phenyl quinazlin-4(3H) one by Volvox aureus at wavelength 260 nm were 76.45%, 83.38% and 83.40% after 3, 5 and 7 days. The percentage of degradation of 2-phenyl 3,1 benzexazin 4-one by V. aureus at wavelength 284 nm was 50.3% after 3 days, 53.23% and 53.24% after 5 and 7 days of incubation, respectively (Table 3). |
| Discussion |
| The microbial degradation of phenols, mainly by bacteria and fungi, has been extensively studied both experimentally and theoretically, but only relatively recently the capabilities of some algae for phenols biodegradation gained interest [20]. The enzmology of the degradation of phenol by Ochromonas danica was previously investigated by [21]. Specific activities for the hydroxylation of phenol to catechol which was oxidized by different isolated algae and make shift in the wavelength at 386 which similar to the wavelength of catechol, involving a putative phenol hydroxylase could not be found in cell-free extracts. Aromatic rings can be ring - opened by either ortho cleavage or meta cleavage after the formation of a dihydroxybenzoidal moiety. Enzymes of both ortho-and meta cleavage pathways were commonly found in bacteria using extracts of phenol-induced cells [22]. The algae were found to cleave catechol in the 2-3 position resulting in the formation of 2- hydroxymuconic semialdehyde. The alga extracts also oxygenated 3- methyl catechol, 4- methyl catechol, 4- bromo catechol and 4-fluoro catechol to the corresponding ring-cleavage products which showed the expected shifts in spectral peaks under acid and alkaline conditions. Inhibition studies using 4- isopropyl catechol [23] and 3- chlorocatechol gave results correspond to those of bacterial studies, showing irreversible and suicide inhibitions, respectively. Specific activities in these extracts for catechol 1.2- dioxygenase (catechol: oxygen 1.2 oxidoreductase were found to be negligible [24]. |
| Biodegradative processes may be carried out by a single microbial species in pure culture or, more often may require the efforts of a mixture of microbes, termed a consortium. The results of this investigation confirm the results of the oxidation of phenol by different isolated algae and resulted in shift in the wavelength at 386 nm which represent the wavelength of catechol. |
| The present results suggest also that the degradation of polycyclic and heterocyclic aromatic compounds by algae seems to be related to the molecular structure of the compound. The compound degradation is related to the physiological metabolism of the algae. |
| There is now evidence that at least four enzyme systems in algae are capable of catalyzing the transformation of benzo pyrene. The catalytic activity of some oxidoreductases in the transformation of PAHs was studied in preliminary investigations, where benzo pyrene was exposed to some enzymes and enzyme preparations. The activity of two representatives of the proposed active enzymes was previously experimentally tested in water solution of benzo pyrene. O-diphenol oxidase was separated from algae and potato tubers. In the presence of this enzyme the rate of degradation of the target carcinogen was essentially increased [10,25]. The results of this investigation showed that the degradation of naphthalene and anthracene was increased by incubation with algae. |
| Vogel and Grbic-Galic [26] obtained evidence suggesting that benzene derivatives could be metabolized under anaerobic conditions through an oxidation pathway rather than a reduction pathway. The initial step of ring oxidation instead of ring reduction appeared to contradict reductive pathway theory [9]. Vogel and Grbic-Galic [26] argued that the anaerobic transformation of benzene and toluene to CO2 and CH4 was preceded by a hydroxylation reaction. Phenol and cresol were identified as intermediates from benzen and toulene, respectively. Vogel TM et al. [26,27] published the reductive pathway for anaerobic metabolism of aromatic compounds. This pathway was believed to be common in all microorganisms involved in aromatic metabolism, including denitrifers, sulfate reducers, and fermenters. The results of this investigation in agreement with the previous results in the manner that the degradation was occurred by different ways such as the oxidation of phenol by algae. |
| Erickson and Fan [28] have summarized information on anaerobic biodegradation of a number of aromatic compounds by means of photometabolism, anaerobic respiration using nitrate or sulfate, and methanogenic fermentation [29,30], however, they proposed hydroxylation as the initial step in naphthalene biotransformation under sulfate- reducing conditions. Bauer JE and Capone DG [31] performed a laboratory study on the degradation of anthracene and naphthalene by the microbiota. The results of this investigation are in accordance with the degradation of naphthalene and anthracene by different algae. Bauer JE and Capone DG [32] evaluated the effects of four aromatic compounds including two PAH, anthracene and naphthalene, on two microbial activities common in oxic and anoxic coastal marine sediments. |
| The obtained results showed that aromatic hydrocarbons were efficiently removed by the biological treatment. Biodegradation achieved a high degree of purification of the polluted water in the axenic cultures. It can be concluded that the degradation of different polycyclic and heterocyclic aromatic compounds by different algae depend on the molecular structure of the compound and physiological metabolism of the alga. The results of this investigation also indicated that when the ratio of degradation was low the compound might be consumed by cells and or absorbed on the wall, and the remainder was degraded by algae. |
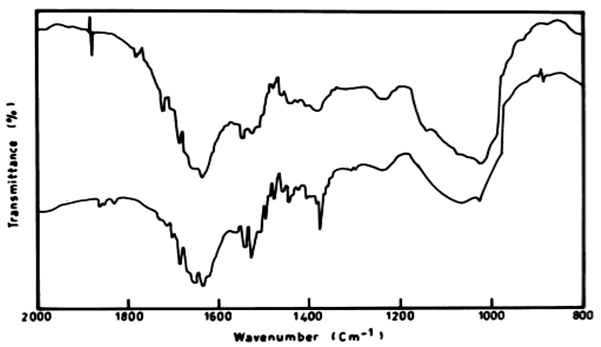
| There was also a difference in the IR peaks of the biomass of L. lagerlerimi before and after treatment by anthracene. There was low ratio reached 26.29% after 7 days and this may be due to the ability of the algae to consume some of anthracene in the biomass and the remainder was degraded, and these results are in agreements with the results obtained by [33]. The infrared absorption regions of polynuclear hydrocarbons include many of those characteristic of the benzene compounds. The bands for C=C are 1625-1600 cm-1, 1590-1575 cm-1 and 1525-1474 cm-1. |
| The infrared spectrum of anthracene before and after growing E. viridis on medium containing anthracene recorded stretching vibration of anthracene at 1620 cm-1. It is also evident that the intensity of the peak at 1620 cm-1 after treatment by E. viridis was diminshed than that before treatment. This might be due to the decrease of the concentration of anthracene after treatment by E. viridis as a result of degradation. The infrared absorption regions of polynuclear hydrocarbons include many of those characteristic of the benzene compounds. The bands for C=C (in-plane vibration) are 1625-1600 cm-11590-1575 cm-1 (v) and 1525- 1474 cm-1 (v). There is region 1000-650 cm-1, and strong band at 750 cm-1. This is due to four adjacent hydrogens (terminal benzene rings), on the other hand, the three peaks also show a strong band at 900 cm-1, which is due to para-hydrogen atoms (Figure 10). |
| In conclusion this work was an attempt to exploit algae to degrade or discolourize phenolic compounds, polycyclic and heterocyclic aromatic compounds. The results showed the ability of algae to degrade or discolourize high amounts of these pollutants via different ways, either by reduction, oxidation or by induction of some enzymes that degrade these toxic compounds. More work should be done in order to further understand the mechanism(s) of degradation and the enzyme system(s) that algae use to degrade such pollutants. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
 |
 |
 |
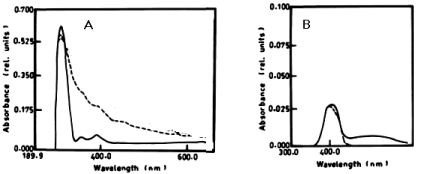 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
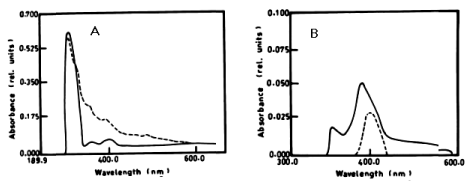 |
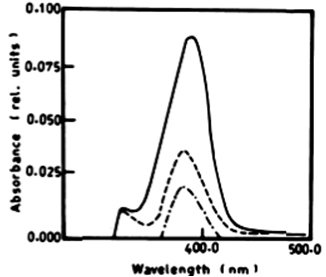 |
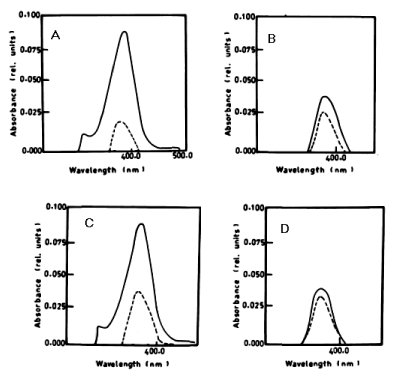 |
 |
 |
| Figure 6 | Figure 7 | Figure 8 | Figure 9 | Figure 10 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 18513
- [From(publication date):
January-2012 - Nov 19, 2025] - Breakdown by view type
- HTML page views : 13246
- PDF downloads : 5267
