Make the best use of Scientific Research and information from our 700+ peer reviewed, Open Access Journals that operates with the help of 50,000+ Editorial Board Members and esteemed reviewers and 1000+ Scientific associations in Medical, Clinical, Pharmaceutical, Engineering, Technology and Management Fields.
Meet Inspiring Speakers and Experts at our 3000+ Global Conferenceseries Events with over 600+ Conferences, 1200+ Symposiums and 1200+ Workshops on Medical, Pharma, Engineering, Science, Technology and Business
Research Article Open Access
Decolorization of Textile Dye Remazol Black B by Pseudomonas aeruginosa CR-25 Isolated from the Common Effluent Treatment Plant
| Jiss Joe1, R. K. Kothari2, C.M. Raval1,2, C.R. Kothari2, V. G. Akbari1 and S. P. Singh1* | |
| 1Department of Biosciences, Saurashtra University, Rajkot, India- 360 005 | |
| 2Christ College, Rajkot, India- 360 005 | |
| Corresponding Author : | Dr. S. P. Singh Department of Biosciences Saurashtra University, Rajkot, India- 360 005 Tel: + 91 281 2586419 Fax: + 91 281 2586419 E-mail: satyapsingh@yahoo.com, satyapsingh125@gmail.com |
| Received March 18, 2011; Accepted June 01, 2011; Published June 13, 2011 | |
| Citation: Joe J, Kothari RK, Raval CM, Kothari CR, Akbari VG, et al (2011) Decolorization of Textile Dye Remazol Black B by Pseudomonas aeruginosa CR- 25 Isolated from the Common Effluent Treatment Plant. J Bioremed Biodegrad 2:118. doi:10.4172/2155-6199.1000118 | |
| Copyright: © 2011 Joe J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Rapid industrialization and urbanization have resulted in large amount of wastes into the environment causing major pollution problem. Among many pollutants, textile industry effluents are the major source of environmental pollution. Synthetic dyes are widely used in the textile, pharmaceutical, cosmetic, and food industries. However, at least 10-15% of the dyes used in textile processing are released into wastewater.
| Keywords | |||||||||||||||
| Effluent treatment; Remazol black B; Dye decolorization; Dye degradation; Pseudomonas aeruginosa | |||||||||||||||
| Introduction | |||||||||||||||
| Rapid industrialization and urbanization have resulted in large amount of wastes into the environment causing major pollution problem. Among many pollutants, textile industry effluents are the major source of environmental pollution. Synthetic dyes are widely used in the textile, pharmaceutical, cosmetic, and food industries. However, at least 10-15% of the dyes used in textile processing are released into wastewater [1]. These effluents are a serious concern because of their adverse effects on many organisms and ecosystem as a whole. Even a minimal concentration of dye is highly visible and can be toxic to aquatic organisms [3], Azo dyes are the largest group of dyes used in industry [4,5], representing more than half of the annual production state by [6]. Azo dyes are characterized by the presence of one or more azo groups -N = N - [7], which are responsible for their colour. When this bond is broken, the compound loses its color [8]. Azo dyes are the largest and most versatile class of dye but due to their structural properties, they are not easily degradable under natural conditions [9] and thus, are not typically removed from water by conventional wastewater treatment systems [10]. Azo dyes resist chemical and microbial attacks [4] and are stable in light and under washing conditions [9]. Compared with chemical/physical methods, biological processes have received much attention due to cost effectiveness, lower sludge production and environmental friendliness [6,11]. Various white rot fungi were shown to decolorize azo dyes using peroxidases or laccases with high oxidative capacities, which oxidize the azo dyes to form compounds of lower molecular weights [12]. However, fungal treatment of effluents is usually time-consuming [11] and aerobic treatment of azo dyes with bacteria usually achieved low efficiencies due to oxygen being a more efficient electron acceptor than azo dyes. Under static or anaerobic conditions, bacterial decolorization is quite effective. Anaerobic processes are usually non-specific with regard to the organisms and dyes [6]. Colorless aromatic amines produced under anoxic conditions during the metabolism of azo dyes may also show toxic, mutagenic, or carcinogenic effects [13]. The azo dye is reduced to the corresponding colorless aromatic amines, which are in turn metabolized with relative ease under aerobic conditions [14]. In this study, we isolated and identified a Pseudomonas species that decolorizes reactive azo dyes. The general characteristics, decolorization efficiency under different physical and nutritional parameters were investigated and its potential use in industrial applications is discussed. | |||||||||||||||
| Materials and Methods | |||||||||||||||
| Chemicals | |||||||||||||||
| The textiles dye Remazol Black B (Commercial purity 85%) (λmax 595nm) was procured from dye manufacturing industries located in Ankelshwar (Gujarat, India). The medium components and other chemicals were of analytical grade. | |||||||||||||||
| Medium | |||||||||||||||
| Mineral Salt Medium (MSM) contained (gl-1): Na2HPO4, 3.6; KH2PO4, 1.0; (NH4)2SO4, 1.0; MgSO4.7H2O, 1.0; CaCl2 .2H2O, 0.1; Fe (NH4) citrate, 0.01, and 1.0 ml of trace element solution. | |||||||||||||||
| The trace element solution had the composition (mgl-1) ZnSO4.7H2O,10; MnCl2.4H2O, 3.0; CoCl2.6H2O,1.0; NiCl2.6H2O, 2.0; Na2MoO4.2H20, 3.0; H3BO3, 30.0; and CuCl2.2H2O, 1.0. The pH of the medium was adjusted to 7. | |||||||||||||||
| Glucose, Peptone, Yeast Extract (GPY) medium contained (gl-1): Glucose, 1.0; Yeast Extract, 5.0; Peptone, 5.0. The pH of the medium was adjusted to 7. | |||||||||||||||
| Isolation and identification of dye decolorizing microorganism | |||||||||||||||
| The dye-decolorizing bacterial strain was isolated from activatedsludge of the common effluent treatment plant, Jetpur, Rajkot, (Gujarat, India). Screening of microorganisms for dye decolorization was carried out on GPY agar plates. After successive streaking, single isolated colony on GPY plate was obtained. | |||||||||||||||
| The bacterium CR-25 was identified by 16S rRNA gene sequence analysis. Genomic DNA was isolated from the pure culture pellet. With consensus primers, the ∼1.5 kb 16S rDNA fragment was amplified using high-fidelity PCR Polymerase. The PCR product was cloned and plasmid DNA was bi-directionally sequenced using the forward, reverse and an internal primer. Sequence data was aligned and analyzed for finding the closest homologous for the microbe. | |||||||||||||||
| PCR conditions | |||||||||||||||
| DNA: 1.0µl, Taq Buffer A (10X) : 2.5µl, dNTP mix (2.5mM each) : 1.0µl, Forward Primer : 1.0µl, Reverse Primer : 1.0µl, Taq DNA polymerase enzyme : 3U, Glass distilled water : to make up the volume to 25µl | |||||||||||||||
|
|||||||||||||||
| Culture preservation and maintenance | |||||||||||||||
| The stock culture was preserved in glycerol at -20°C and used for decolorization and degradation studies after pre-culturing in GPY medium. | |||||||||||||||
| Decolorization study and UV –visible spectral analysis | |||||||||||||||
| GPY broth containing 100 ppm RBB was examined under static conditions. A stationary-phase culture grown under aerobic conditions at 37°C was used as a stock culture. GPY broths were inoculated with 1 ml (1.115 x 109 cells. ml-1) stock culture and incubated under static. The absorbance and decolorization rate were determined at 0, 24, 48 and 72 h after incubation. Culture aliquots were centrifuged at 12,000 X g for 10 min. The supernatant filtrates were scanned with a range of wavelengths (λ, 200 to 800 nm) on UV/Visible Spectrophotometer (Shimadzu UV 1600). The decolorizing activity was expressed in terms of percentage decolorization. Decolorization of dyes was determined by monitoring the decrease in absorbance at the maximum wavelength of dye (λmax 595nm). The un-inoculated GPY supplemented with dye was used as control. An UV-Visible scanning spectrophotometer (Shimadzu UV 1600) was used for absorbance measurement. Decolorization rate was calculated as the following, | |||||||||||||||
| Decolorization rate (%) = (A-B)/A X 100 | |||||||||||||||
| A = Initial absorbance | |||||||||||||||
| B = observed absorbance | |||||||||||||||
| Decolorization Index (Di): To correlate the decolorization efficiency with biomass, decolorization index were determined. | |||||||||||||||
| Decolorization Index (Di) = Decolorization (%)/Biomass (mg.l-1) | |||||||||||||||
| Effects of static and shake flask condition on decolorization of RBB: To examine the effect of static and shaking condition on decolorization, GPY broth containing 100 ppm RBB was examined in static and shaking condition. GPY broths were inoculated with 1 ml (1.115 x 109 cells.ml-1) stock culture and incubated under static and shaking (120 rpm) conditions. The absorbance and decolorization rate were determined at 0, 24 and 48 h after incubation. | |||||||||||||||
| Effects of dye concentration on decolorization of RBB: Various concentrations of RBB (50-500 ppm) were prepared in GPY broth. GPY broth inoculated with stock culture and incubated under static condition. After incubation, the absorbance and decolorization rate were determined at 0, 24, 48 and 72 h after incubation. | |||||||||||||||
| Effects of carbon and nitrogen source on the decolorization of RBB: MSM containing RBB (100 mgl-1) and the different carbon sources (Glucose, sucrose, maltose, fructose, raffinose) nitrogen sources (yeast extract and peptone) at 0.2% were prepared. The medium was inoculated with stock culture. Culture aliquots were harvested at 0, 24, 48 and 72h of growth and analyzed for decolorization. To examine the effect of nitrogen source, MSM broth with glucose (pH 7) containing 100 ppm RBB and 0.2% of nitrogen source (ammonium nitrate, ammonium chloride, ammonium oxalate, ammonium sulphate, ammonium phosphate, ammonium ferrous sulphate, glycine, urea, ammonium molybdate, sodium nitrate, ammonium acetate, a-naphtholamine, peptone and yeast extract) was inoculated with stock culture and incubated in static condition. The absorbance and decolorization rate were determined. | |||||||||||||||
| Effects of pH on the decolorization of RBB: GPY media containing 100 ppm dye were prepared in different buffer solutions. The buffers selected for media preparation were 0.1 M citrate buffer (pH 5), 0.1 M sodium phosphate buffer (pH 6 and 7) and 0.1 M Tris buffer (pH 8 and 9). Fresh GPY broths were inoculated with stock culture and incubated under static conditions. The absorbance and decolorization rate were determined at 0, 24, 48 and 72h after incubation. | |||||||||||||||
| Effects of Size of inoculum on decolorization of RBB by Pseudomonas aeruginosa CR-25: Sixteen-hour old culture of P. aeruginosa CR-25 growing in CMB was used as inoculum. Twenty five flasks containing 100 ml of sterile CMB medium (100 mgl-1) RBB were prepared. In a set of five flasks, each flask was inoculated with a fixed inoculum size varying between 0.2-1% and incubated at 37°C. Inoculated and un-inoculated controls were also included. The cultures were harvested at an interval of 12 h and analyzed for percent decolorization. | |||||||||||||||
| Effects of temperature on the decolorization of RBB: GPY medium containing 100 ppm RBB were inoculated with stock culture of Pseudomonas aeruginosa CR-25. These were incubated at 0, 20, 30, 37, 40 and 70°C under static conditions. The absorbance, Di and decolorization rate were determined at 0, 24, 48 and 72h after incubation. | |||||||||||||||
| Effects of salt on decolorization of RBB | |||||||||||||||
| GPY medium with varied salt (NaCl) concentrations (0%, 0.5%, 2%, 4%, 5%, 6%) were inoculated with 1% stock culture of P. aeruginosa CR-25 incubated in static condition at 37°C. Samples were harvested at an interval of 0, 24, 48 and 72 h and analyzed for absorbance and decolorization rate. | |||||||||||||||
| Effect of Sequential static and shaking condition on decolorization | |||||||||||||||
| 250-ml Erlenmeyer flasks containing 100 ml GPY with (100 mgl-1) RBB were inoculated with 1% stock culture of P. aeruginosa CR-25 and incubated under static conditions for 24 h at 37°C. After 24 h, the same culture flask was incubated under shake flask conditions for further 24 h. Inoculated and uninoculated controls were also included. Samples were harvested at specified time intervals and analyzed for decolorization. Chemical Oxygen Demand (COD) was measured by the procedures outlined in Standard Methods (American Public Health Association, APHA, 2002). | |||||||||||||||
| Data analysis: Values quoted in this paper are reported as the average of two sets of results that were obtained from the same sample. The experiments were performed in triplicate and average values with standards errors were computed. | |||||||||||||||
| Results and Discussion | |||||||||||||||
| Identification of bacterial isolate | |||||||||||||||
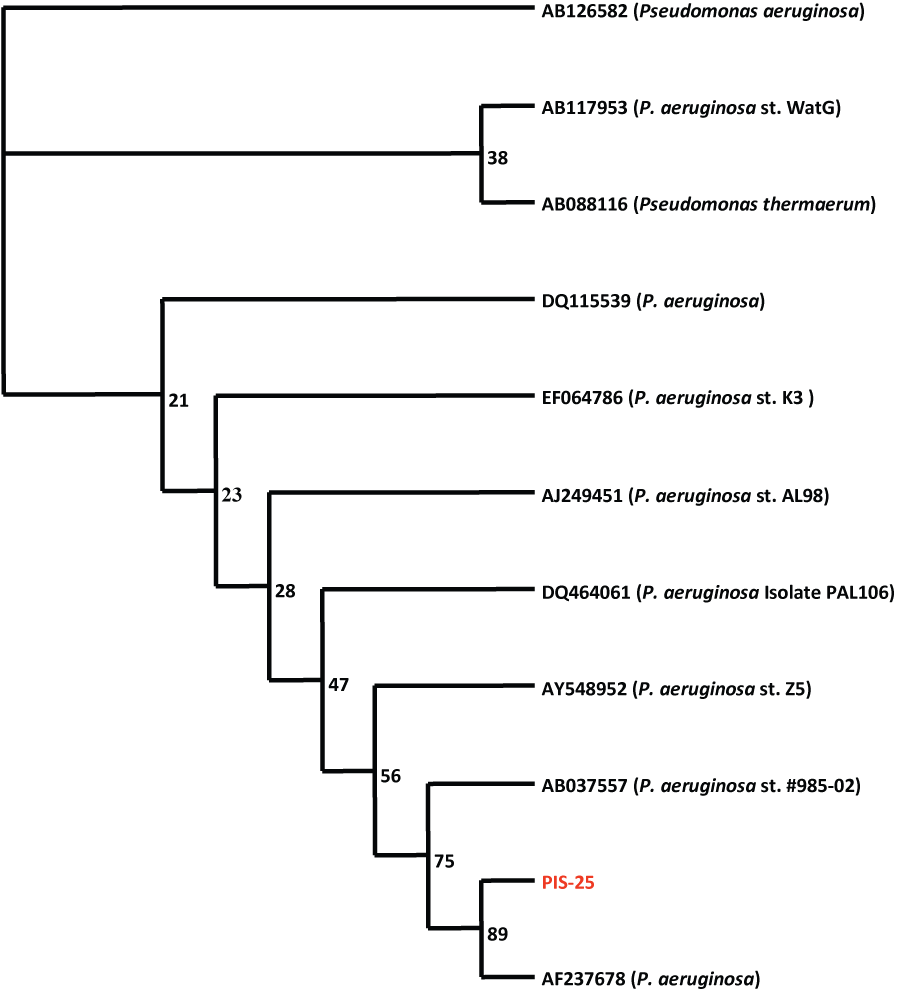
| Based on 16S rRNA gene nucleotides homology and phylogenetic analysis, the bacterium, CR-25 (Gene Bank Accession Number: AF237678) was identified as Pseudomonas aeruginosa. | |||||||||||||||
| Genomic DNA was isolated from the pure culture and using consensus primers, the ∼1.5 kb 16S rDNA fragment was amplified using TaqDNA Polymerase. The PCR product was bi-directionally sequenced using the forward, reverse and an internal primer and sequence data was aligned and analyzed for finding the closest homologs for the microbe. The phylogenetic tree of Pseudomonas aeruginosa CR- 25 (Figure 1) shows that the nearest homologous species was found to be Pseudomonas thermaerum (Accession No. AB088116). Information about other closely related bacteria is evident from the Alignment score (table 1). | |||||||||||||||
| Decolorization study and UV –visible spectral analysis | |||||||||||||||
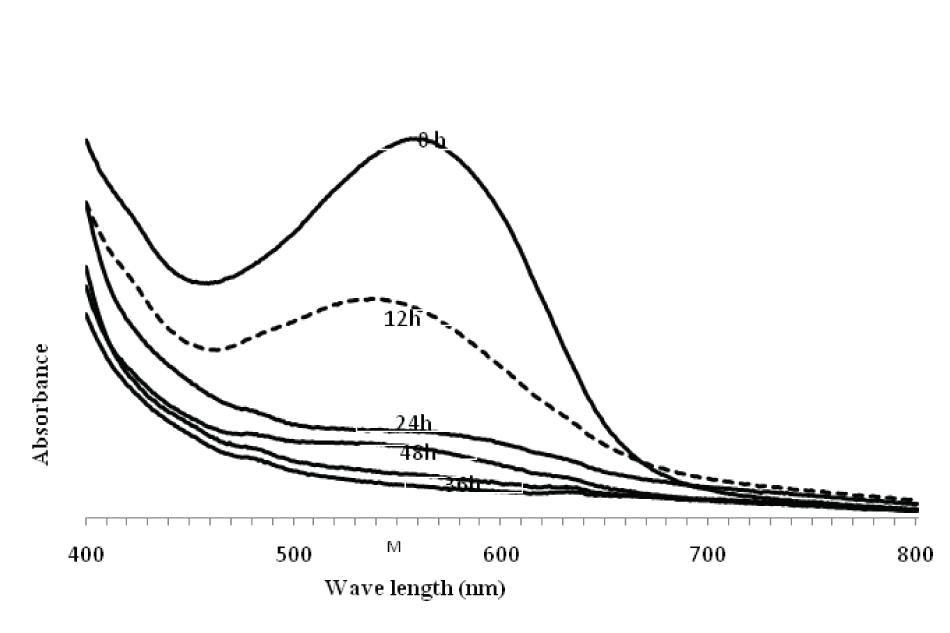
| Spectral analysis of RBB (λmax 595nm) dye was carried out in the range of 200-800 nm. Results on the degradation revealed that the dye peak in the visible region gradually decreased and finally disappeared with increasing incubation time. The abiotic control showed no change in color content (data not shown). This indicated simplification of the native compound (dye) and thus provided strong evidence of the decolorization process (Figure 2). | |||||||||||||||
| Effect of static and shake flask condition on decolorization of RBB | |||||||||||||||
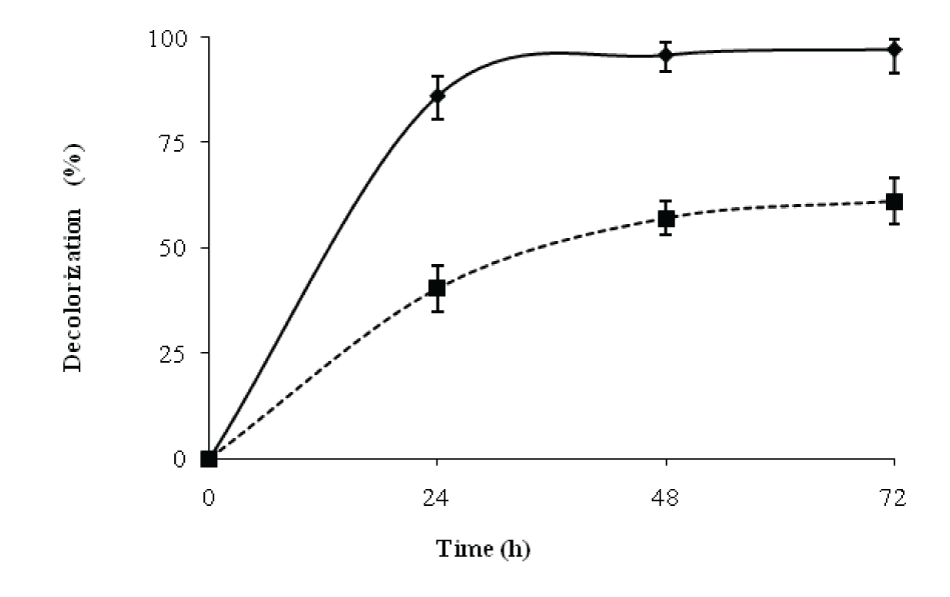
| The decolorization efficiencies of this strain were compared under static and shaking conditions. After 24 h, the decolorization of RBB obtained under static conditions was 86% while under shake flask conditions, the decolorization was 40%. The results (Figure 3) showed that during 24 and 48h, the decolorization activity increased with increase in biomass. During 48-72h; however, there was no significant change in decolorization, while the biomass gradually increased leading to lower Di values after 48h. [15] achieved 67% color removal, while [16]. Achieved 78% color removal. According to a previously reported batch study, the decolourization efficiencies for Reactive Black B were 67 and 73% color removal in 10.5 and 79 h respectively [17]. Similarly, in another study, a 66% decolourization was observed in 24 h [18] Therefore, the results reported in our studies are quite comparable to the previous works in literature [15] for the decolorization of remazol black B. | |||||||||||||||
| Effect of sequential static and shaking conditions on decolorization | |||||||||||||||
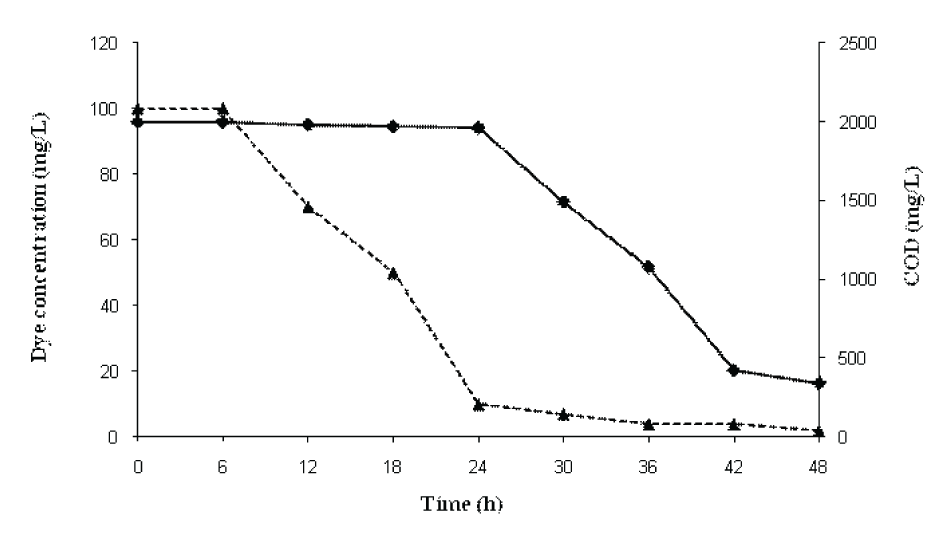
| Decolorization of RBB and reduction in COD during sequential static-shaking culture of P. aeruginosa CR-25 is shown in (Figure 4). The results showed that majority of the color were removed in the static phase (anaerobic) while the COD was significantly reduced under shaking (aerobic) conditions. The initial COD of the medium consisting of 100mgl-1 RBB was 2000 mgl-1. After the treatment with P. aeruginosa CR-25 for 24h, the COD was only marginally reduced to 1962mgl-1 with 90% dye decolorization. After 24h, the decolorized sample was subjected to shake flask conditions and COD was measured at 30, 36, 42 and 48h. The COD values were correspondingly reduced to 1490, 1080, 422 and 340mgl-1 at 30, 36, 42 and 48h, respectively. There was regular reduction in COD after every 6 h under shake flask conditions and finally it was reduced to 340mgl-1. The COD of medium control was 325mgl-1. Thus, the COD load exhibited due to RBB was completely removed during shake flask culture conditions. | |||||||||||||||
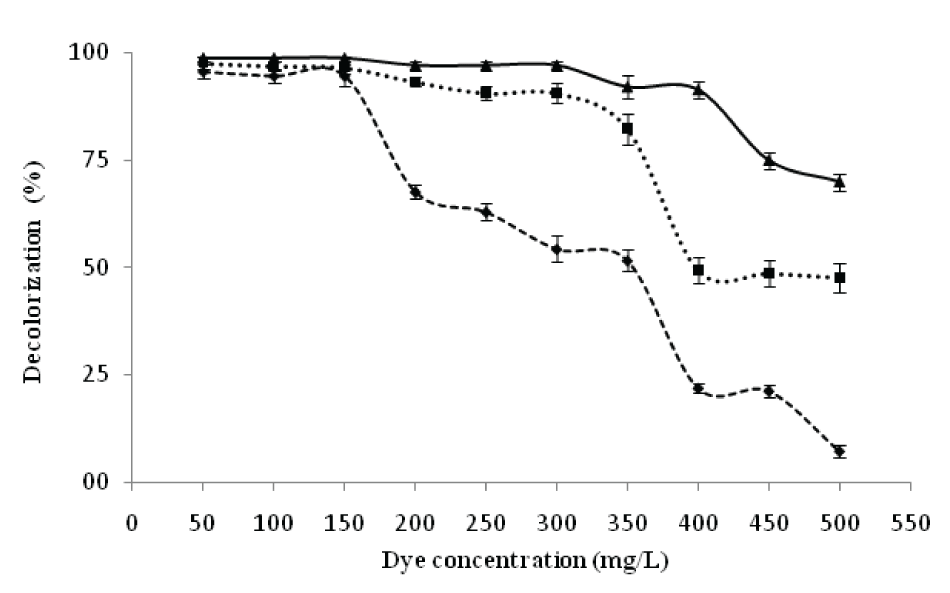
| Effect of dye concentration on decolorization of RBB | |||||||||||||||
| Decolorizing activity of P. aeruginosa CR-25 was studied using RBB at different initial concentrations varying from 50 to 500 mgl-1 (Figure 5).The results were obtained during 24 h of incubation. The decolorization up to 96, 95, and 95% at initial RBB concentrations of 50, 100 and 150 mgl-1 respectively, was obtained. At 200, 250, 300 and 350 mgl-1 concentrations, however, the decolorization were reduced to 68, 65, 57 and 52% respectively. Earlier, a 67% decolorization with an initial RBB concentration of 500mgl-1 under anaerobic culture conditions was reported [15]. | |||||||||||||||
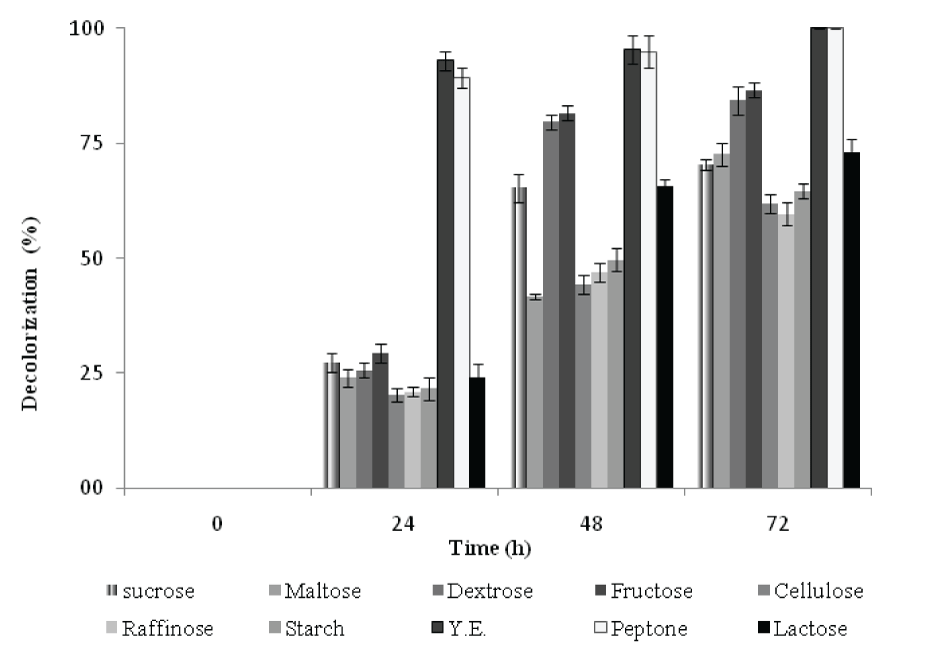
| Effect of carbon and nitrogen source on the decolorization of RBB | |||||||||||||||
| The decolorization activity of RBB by P. aeruginosa CR-25 growing in MSM with selected carbon sources under static culture condition was monitored for 72h. Except Yeast extract and peptone, the decolorization of RBB was in the range of 20 - 29% (Figure 6) after 24h incubation. In the presence of Yeast extract, the decolorization reached to 96%. The trend of Di in the presence of fructose and maltose resembled to that with yeast extract and peptone. However, there was no increase in decolorization activity during 24h. This indicated increase in biomass without significant increase in decolorization activity. In the presence of other carbon sources, Di values increased with increasing decolorization activity during 24 and 48h. On further incubation (48-72h), while, there was no significant increase in the decolorization activity, the Di values decreased with incubation time. | |||||||||||||||
| The organisms decolorized 8-41% dye in the initial 24h, in the presence of all selected nitrogen sources except yeast extract and peptone (data not shown) In the presence of peptone decolorization was achieved up to 77%, while medium supplemented with yeast extract showed 91% decolorization (Data not shown). During 24h, the decolorization of RBB in the presence of yeast extract (91%) and peptone (77%) with Di 209 and Di 171, was achieved, respectively. After 48h, there was no considerable increase in decolorization activity, but Di gradually decreased. The Di values in the presence of ammonium acetate and urea corresponded to those obtained for of yeast extract and peptone. In the presence of other nitrogen sources, the Di increased with increase in decolorization activity during 24 and 48h. Further, there was no noticeable increase in decolorization activity during 48-72h; however, Di decreased with incubation time. | |||||||||||||||
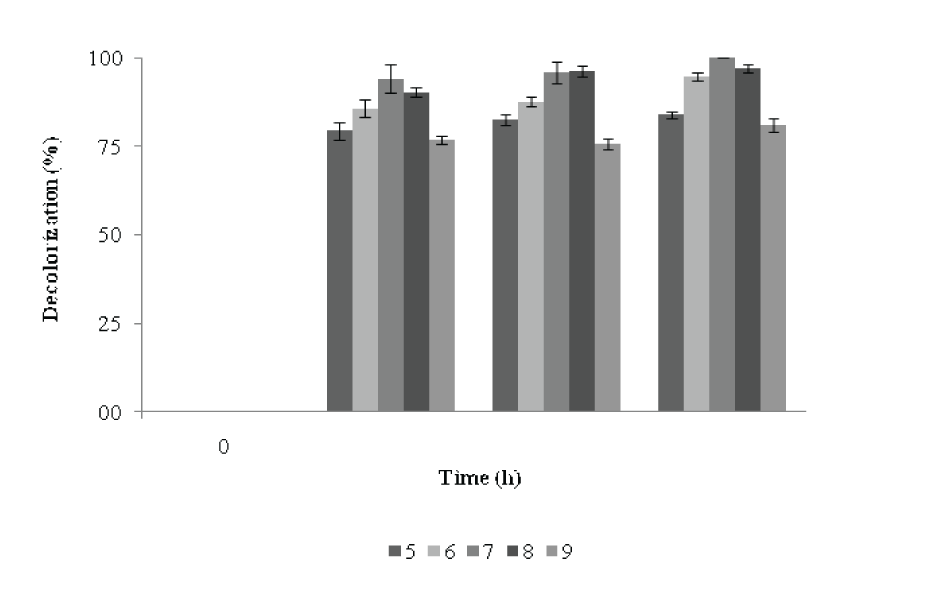
| Effects of pH on the decolorization of RBB | |||||||||||||||
| The decolorization efficiencies of this strain were compared across a range of pH values. The optimal pH for complete decolorization of RBB by P. aeruginosa CR-25 was 7. The dye was decolorized in the range of pH 5 to pH 9. After 24h of incubation, at pH 6 and 5, the decolorization was 86 and 79%, respectively which after 72h of incubation reached to 95 and 85% with biomass of 0.615 A660 and 0.551 A660 respectively (Figure 7). At pH 8, a 90% decolorization activity was recorded after 24 h which reached to 97% at 72 h with biomass of 0.638 A660. A noticeable decolorization activity and increase in biomass was observed at pH 9 after 72 h of incubation. | |||||||||||||||
| Ionic strength plays an important role for enzyme regulation. Figure 4 illustrates the change in biomass with decolorization in response to various pH. At pH 7 and 8, the decolorization of RBB was achieved to the extent of 94 -90%, while the Di was 161 and 224, respectively during 24h of incubation. At pH 7, maximum decolorization was achieved with lower Di values compared to others pH. At pH 8, the maximum Di was achieved. After 48h, there was no significant change in decolorization activity, but Di had a decreasing trend. At the end of 48h, at pH 9, the Di value was maximum which decreased during 48-72h. At pH values, the Di increased with increase in decolorization activity during 24h which then decreased during 48 to 72h, indicating towards increase in biomass without a significant increase in decolorization activity. There was not much increase in decolorization activity during 48-72h, but Di decreased with incubation time. The pH tolerance was similar to that observed for the decolourization of azo dyes in halophilic and halotolerant bacteria [19]. However, the pH range was wider as compared to those reported for other decolorizing bacteria [20]. The feature of decolorization under alkaline conditions suggests nobility for industrial applications, as processes using reactive azo dyes are performed under alkaline conditions [22]. | |||||||||||||||
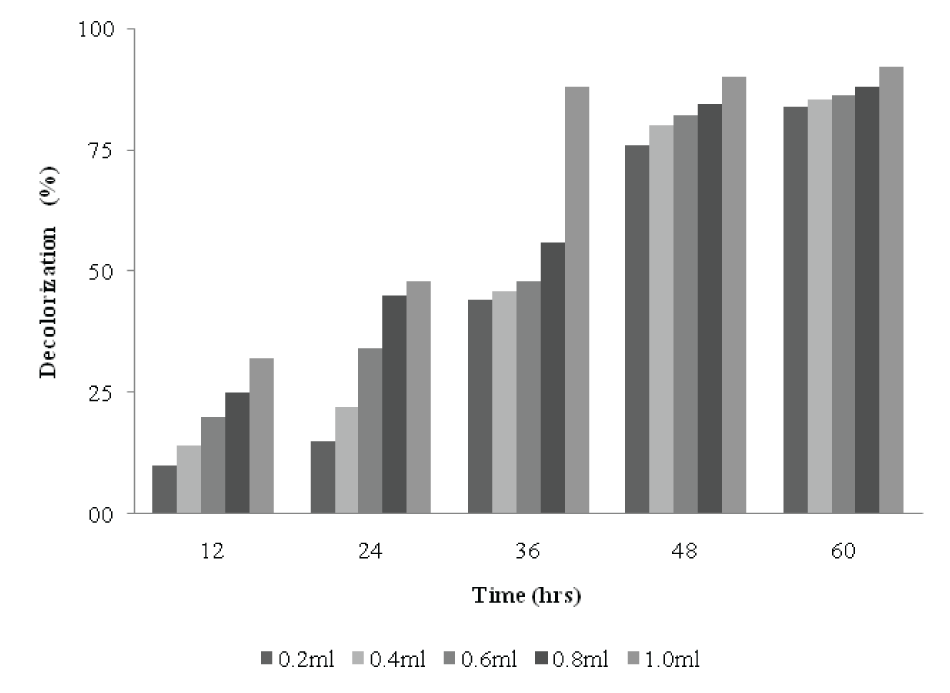
| Effects of size of inoculum on decolorization | |||||||||||||||
| Figure 8 shows the effects of size of inocula on decolorization of dyes. When the size of inoculum was 0.2 ml, 15% decolorization was obtained within 24h. However, between 36 to 48h, a 36% decolorization was achieved. A 22% decolorization was obtained within 24h with 0.4 ml inoculum size and 80% after 48h. The dye was rapidly decolorized in the flasks to which 1 ml inoculum was added. As the inoculum size increased from 0.6-0.8 ml, there was increase in decolorization. The decolorizing activity increased with increase in the inoculum size, reaching (88%, at 1% of inoculum) after 36h, beyond which, decolorization did not vary significantly. It can be concluded that under unlimited supply of dye and medium, if there is increase in the size of inoculum, the decolorization increases proportionately. However, in contrast to this observation, there was no proportionate increase in decolorization with increasing size of inoculum for Kurthia sp. [2]. | |||||||||||||||
| Effects of temperature on the decolorization | |||||||||||||||
| Microbial growth is temperature specific and temperature is an important physical parameter that influences the cell metabolism. Variation in temperature decreases or increases the enzyme activity. Dye decolorization and degradation is an enzyme catalyzed reaction and thus, the rate will depend on the amount of dye decolorized. | |||||||||||||||
| After 24h of incubation, the maximum decolorization (94%) was obtained at 37°C. At 40°C and 50°C, the decolorization reduced to 26% and 5% respectively. At 60°C and 70°C, the decolorization of RBB was completely inhibited. Inhibition in decolorizing activity at higher temperatures can be attributed to the loss of cell viability or to the denaturation of azoreductase enzyme [23]. Wong & Yuen reported that Klebiella pneumoniae RS-13 decolorized of Methyl red in the temperature range of from 23 to 37°C, whereas, at 45°C decolorization was completely inhibited. Pseudomonas sp. reportedly decolorized Malachite green, Fast green, Brilliant green, Congo red and Methylene blue optimally at 37°C [25]. | |||||||||||||||
| Effects of salt concentration on the decolorization | |||||||||||||||
| Due to the lack of detailed studies on biological decolorization in high salt-dye wastewater, it is difficult to evaluate the biological treatment. The experiments were carried out to study the potential of P. aeruginosa CR-25 for the salt-tolerance, which can be useful in the treatment of high-salinity colored wastewater [26]. P. aeruginosa CR-25 was able to grow up to 6% NaCl. The growth was inhibited at higher salt concentration. A significant decolorization was achieved in presence of all salt concentrations selected. At 0.5%, 2%, 4% salt concentrations, the decolorization of RBB were 66%, 65% and 63% respectively after 48 h of incubation. In the presence of 5 and 6% salt, the decolorization activity was affected to some extent at the end of 48h. Beyond 48 h, there was no increase in decolorization. The positive findings suggested that there might be a simple process for the high salt wastewater treatment, which could be incorporated into conventional process with the use of such potential isolates. | |||||||||||||||
| Removal of COD during decolorization of RBB by P. aeruginosa CR-25 | |||||||||||||||
| Decolorization of RBB and reduction in COD during sequential static-shaking culture of P. aeruginosa CR-25 is shown in (Figure 4). The results showed that majority of the color were removed in the static phase (anaerobic) while the majority of COD was reduced under shaking (aerobic) conditions. The initial COD load of the reaction flasks consisting of 100mgl-1 RBB was 2000mgl-1. When it was treated with P. aeruginosa CR-25, after 24h of incubation the COD was only marginally reduced to 1962 mgl-1 with 90% dye decolorization. After 24h, the decolorized sample was subjected to shake flask conditions and COD was measured at 30, 36, 42 and 48h. The COD values were correspondingly reduced to 1490, 1080, 422 and 340 mgl-1 at 30, 36, 42 and 48h. It was observed that there was regular reduction in COD after every 6h under shake flask conditions and finally reduced to 340mgl-1. The COD of medium control was 325mgl-1. Thus, the COD load exhibited due to RBB was completely removed during shake flask culture conditions. | |||||||||||||||
| Conclusion | |||||||||||||||
| P. aeruginosa CR 25 isolated from textile dye waste water achieved a significant decolorization of RBB. Among many bacterial strains capable of decolorizing RBB, P. aeruginosa CR 25 showed maximum decolorization within short time. The organism could grow in simple, inexpensive medium and was able to effectively decolorize (97%) the textile dye RBB in the presence of peptone and yeast extract. One of the attractive features of this strain was its ability to grow and decolorize the dye in the presence of 6% NaCl. | |||||||||||||||
| Decolorization of RBB under anaerobic conditions by using facultative microorganism P. aeruginosa CR 25 was quite effective. Anaerobic conditions exhibited better decolorization than aerobic conditons. Almost complete decolorization was obtained with up to 150µg/ml dye within 24 hours. The results as a whole suggest that complete reduction of azo dyes within a reasonable time will be possible provided that suitable nutritional and anaerobic conditions are maintained. At 37°C, pH 7.0, under anaerobic conditions, the strain displayed maximum color removal. At higher concentrations, the decolorization efficiency was, however, inhibited. | |||||||||||||||
| Finally, we can conclude that that P. aeruginosa CR 25 was a potential organism and would be a suitable biological agent for the treatment of environmental pollution caused by textile industries. However, large scale experimentation to assess its field suitability would further be needed. | |||||||||||||||
References
|
|||||||||||||||
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
 |
 |
 |
| Figure 6 | Figure 7 | Figure 8 |
Post your comment
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 18761
- [From(publication date):
June-2011 - Jan 17, 2026] - Breakdown by view type
- HTML page views : 13796
- PDF downloads : 4965
