Make the best use of Scientific Research and information from our 700+ peer reviewed, Open Access Journals that operates with the help of 50,000+ Editorial Board Members and esteemed reviewers and 1000+ Scientific associations in Medical, Clinical, Pharmaceutical, Engineering, Technology and Management Fields.
Meet Inspiring Speakers and Experts at our 3000+ Global Conferenceseries Events with over 600+ Conferences, 1200+ Symposiums and 1200+ Workshops on Medical, Pharma, Engineering, Science, Technology and Business
Editorial Open Access
Biomimetic Hydrogels as Scaffolds for Tissue Engineering
| Junmin Zhu* | |
| Department of Biomedical Engineering, Case Western Reserve University, 10900 Euclid Avenue, Cleveland, Ohio 44106, USA | |
| Corresponding Author : | Junmin Zhu Department of Biomedical Engineering Case Western Reserve University 10900 Euclid Avenue, Cleveland Ohio 44106,USA Tel: +1-216-368-0270 Fax: +1-216-368-4969 E-mail: junmin.zhu@case.edu |
| Received November 19, 2012; Accepted November 21, 2012; Published November 23, 2012 | |
| Citation: Zhu J (2012) Biomimetic Hydrogels as Scaffolds for Tissue Engineering. J Biochips Tiss Chips 2:e119. doi:10.4172/2153-0777.1000e119 | |
| Copyright: © 2012 Zhu J. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Bioengineering and Bioelectronics
| Hydrogels have been widely used as tissue engineering scaffolds due to their good biocompatibility, soft tissue-like properties, and highly water-swollen networks that allow permeability for oxygen, nutrients and water-soluble metabolites [1-3]. They can be fabricated from natural biopolymers, synthetic polymers or their hybrids. Biopolymer-derived hydrogels, such as collagen, fibrin and matrigel, usually have concerns in weak mechanical strength, potential immunogenic reactions and animal virus contamination [2]. To overcome these drawbacks, synthetic hydrogels have emerged as important alternatives because they have a well-defined structure, controlled chemical composition and tunable mechanical property. However, most synthetic materials are bio-inert [4]. To address this issue, more research has been focused on the design of biomimetic hydrogels, which aims to mimic the physicochemical and biological properties of natural materials for tissue engineering [4-6]. |
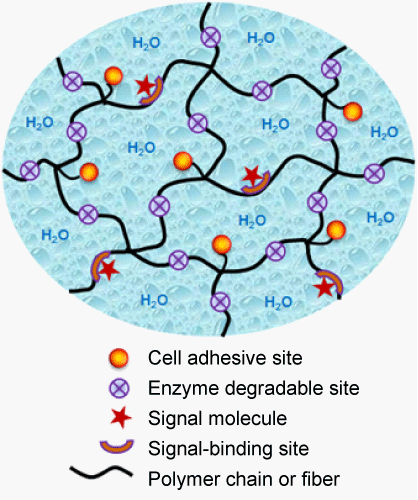
| A desirable scaffold for tissue engineering should have the potential to provide an Extracellular Matrix (ECM)-mimetic microenvironment (Figure 1), in order to specially interact with the surrounding cells or tissues, and guide new tissue formation. The tissue of the human body contains significant extracellular space, into which tissue cells are embedded. Tissue dynamics, such as its formation, function and regeneration after damage, is the result of an intricate temporal and spatial coordination of numerous cellular events mediated by cell/ ECM interactions [7]. The natural ECM is a complex porous and fibrillar structure filled with ECM molecules, including proteins, glycosaminoglycans and proteoglycans, and can provide an ideal microenvironment to support cell growth and tissue remodeling [8]. Cell and matrix biologists have long realized that the natural ECM is an attractive model for designing tissue engineering scaffolds [9,10]. |
| The principle for designing biomimetic hydrogels is to mimic the natural extracellular microenvironment to facilitate the interactions between hydrogels and surrounding cells through molecular recognition, and further enhance specific cellular response and tissue regeneration [4,9]. The mimicry can be chemical or physical.Physical mimicry tries to mimic the porous and fibrillar structure and mechanical property of the ECM, which is essential for cell shape, migration and morphogenesis. Chemical mimicry intends to incorporate bioactive molecules into hydrogels, which is crucial for protein binding and downstream signal pathway. Natural biopolymers, such as proteins, enzymes and polysaccharides are attractive models for the design of biomimetic hydrogels with desired structures, mechanical properties and biological cues. To mimic the porous and fibrillar structure of the ECM, fibrous hydrogel scaffolds have been developed by electrospinning [11] and peptide self-assembly [12]. To incorporate ECM-like bioactivities, a variety of bioactive molecules (e.g. peptides, heparin and growth factors) derived from ECM components have been incorporated into synthetic hydrogels with ECM-mimetic biological properties, such as cell adhesion [13], proteolytic degradation [14] and growth factor-binding [15] (Figure 1). In addition, biomimetic hydrogels are also versatile for microfabrication to fabricate cellencapsulated biochips and tissue chips [16,17]. |
| In summary, biomimetic engineering of synthetic hydrogels is an attractive strategy to develop scaffolds for tissue engineering, which provides fundamental knowledge to understand cell/scaffold interactions, cellular response and tissue formation. The physicochemical and bioactive properties of biomimetic hydrogels can be fine-tuned through variations in the scaffold morphology, crosslinking density, molecular organization and biomolecule incorporation. However, current biomimetic hydrogel scaffolds are still lack of the hierarchical structure and multiple biofunctions of the ECM. An important future work is to mimic the structure, morphology and bioactivity of the ECM as closely as possible, in order to design synthetic hydrogels with an ideal biomimetic microenvironment to support cell growth and tissue regeneration. |
| References |
|
Figures at a glance
 |
| Figure 1 |
Post your comment
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15321
- [From(publication date):
December-2012 - Dec 04, 2024] - Breakdown by view type
- HTML page views : 10706
- PDF downloads : 4615
