| Research Article |
Open Access |
|
| M. S. T. Makki*, R. M. Abdel-Rahman and M.S. El-Shahawi |
| Department of Chemistry, Faculty of Science, King Abdulaziz University, P.O. Box 80203 Jeddah 21589, Kingdom of Saudi Arabia |
| Â |
| *Corresponding authors: |
M.S.T Makki
Department of Chemistry
Faculty of Science, King Abdulaziz University
P. O. Box 80203 Jeddah 21589
Kingdom of Saudi Arabia
Tel: 00-9662-6952000
Fax: 00-9662-6952292
E-mail: mmakki@kaau.edu.sa |
|
| Â |
| Received September 25, 2012; Published November 30, 2012 |
| Â |
| Citation: KMakki MST, Abdel-Rahman RM, El-Shahawi MS (2012) Synthesis and Voltammetric Behavior of Some Novel 1-Acyl/benzoyl-1-anilido-4-methyl-arylbutadiene Derivatives and their uses as Chelating Agents for Trapping of Trace and Ultra Trace Concentrations of Bismuth (III) in Wastewater. 1:501. doi:10.4172/ scientificreports.501 |
| Â |
| Copyright: © 2012 Makki MST, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| Â |
| Abstract |
| Â |
| A series of new 1-acyl/benzoyl-1-anilido-4-methyl-aryl-1,3-butadiene derivatives (II-IV) was prepared by warming 4-aminoantipyrine, 2-amino-5-chloropyridine, 2-amino-5-nitropyridine, 2-aminobenzthiazole, sulfanilamide and/or sulfadiazine with preheated ethyl acetoacetate and/or ethyl benzoylacetate in dry conditions followed by condensation with crotonaldehyde and/or 4-dimethylaminocinnamaldehyde in boiling ethanol-piperidine. The prepared compounds were fully characterized via their elemental analysis and spectroscopic measurements (UV Vis., IR, NMR). The biocidal effects of some prepared compounds (III d, e, f, IV d, e, f) as antimicrobial agents and photochemical probe agents have been investigated. The voltammetric behavior of the two compounds IIa and IVa in N, N-dimethylformamide was investigated. The compound IVa was successfully tested for the removal and or separation of bismuth (III) employing liquid - solid technique involving the use of the solid sorbent polyurethane foams. |
| Â |
| Keywords |
| Â |
| Synthesis; 1-Acyl/benzoyl-1-anilido-4-methyl-aryl-1, 3-butadiene; Biocidal voltammetry; Wastewater |
| Â |
| Introduction |
| Â |
| Recent years have seen an upsurge of interest on the synthesis and spectroscopic characterization of β-diketones as potential ligands [1,2]. Complex formation in this class of compounds is conceived by replacement of the enolic proton by chelation with metal ions in a bidentate fashion [3,4]. The β-diketone 3-salicylidene-2, 4-pentanediones and related compounds have been used successfully as proper chelating agents with a series of many metal ions [5-8]. Knoevenagel condensate of substituted benzylidenes with active methylene compounds were performed efficiently employing ultra stable Zeolite as heterogeneous catalyst [9]. |
| Â |
| Abdel-Rahman et al. [10-14] have reported a series of benzoyl -acetanildes and their physico organic properties. The starting material was used by the same authors for building a series of novel bio-active pyrazoline derivatives have been reported [10,11]. In addition, the thermodynamic characteristics and spectroscopic characterization of a series of compounds namely hydrazono – 1, 3- bi carbonyl derivatives and their lanthanide complexes have been critically investigated [13,14]. |
| Â |
| The chelation behavior of a series of dicarbonyl towards some metal ions has been reported [15-18]. Moreover, metal chelates of β-diones have interesting properties in particular in industrial applications [19-21]. The dimerized species of 2-diazo-3-methyl-1-phenyl-5-pyrazolone produced the compound 4-(5-hydoxy-4-pyrazolxlimino-2-pyrazolin- 5-one. This compound formed complexes with excellent stability towards copper (II) [20]. All these investigations are also indicated that, no work is known on the Knoevenagel condensate β- ketoanilide and its metal chelates. In continuation to our previous work [21-36], the present study is focused on the synthesis and characterization of a series of some β-diketone derivatives bearing anilido moieties and their unsaturated β-diketone derivatives. In view of the voltammetric behavior, one of the prepared compounds was successfully used as a trapping agent for the pre-concentration and determination of bismuth (III) as a highly toxic metal ions in the industrial waste water. |
| Â |
| Experimental |
| Â |
| Apparatus |
| Â |
| A Metrohm 797 VA trace analyzer and 797 VA stand were used for recording the electrochemical experiments. A three-compartment borosilicate (Metrohm) voltammetric cell (10 mL) incorporating hanging mercury dropping electrode (HMDE, drop surface area 0.38 mm PP2PP) as working, double-junction Ag/AgCl, (3M KCl), as a reference and platinum wire (BAS model MW-1032) as counter electrodes, respectively. A Perkin Elmer (Lambda EZ-210) double beam spectrophotometer (190-1100 nm) with 1 cm (path width) was used for recording the electronic spectra of the prepared solutions. Digital pHmeter (model MP220, Metter Toledo) was used for pH measurements with absolute accuracy limits of ±0.1 pH unit defined by NIST buffers. A Perkins Elmer model RXI-FT-IR system 55529 was used for recording the IR spectra of the prepared compounds. A Brucker advance DPX 400 MHz model using TMS as an internal standard was used for recording the 1HNMR spectra of the compounds on deuterated DMSO. A GCMS- QP 1000-Ex model was used for recording the mass spectra of the compounds. Melting points were determined with an electro thermal Bibbly Stuart Scientific Melting Point SMPI (US). Molecular weights of the compounds were preformed on Micro analytical center, Cairo University, Egypt. Microanalysis (Nitrogen %) was performed by microanalytical center Ain-Shams University-Cairo-Egypt. |
| Â |
| Reagents and materials |
| Â |
| Low density polyethylene (LDPE) bottles, Nalgene were used for the collection of the different water samples. LDPE bottles were carefully cleaned first with hot detergent, soaked in 50% HCl (Analar), HNO3 (2.0 mol L-1), subsequently washed with dilute HCl (0.5 mol L-1) and finally rinsed with distilled water. N,N-Dimethylformamide (DMF) and the supporting electrolyte tetraethyl ammonium chloride (TMA+.Cl-) were purchased from BDH chemicals.. The sample solution was stored in LDPE bottles and stored at -20°C. Britton - Robinson buffers of pH 2-11 were prepared from the acid mixture of phosphoric acid, boric acid, acetic acid (0.04 mol L-1) and adjusting the pH to the required value with sodium hydroxide (0.20 mol L-1). Low density polyethylene (LDPE) bottles were used for the collection of the different water samples. LDPE bottles were carefully cleaned first with hot detergent, soaked in 50% HCl (Analar), HNO3 (2.0 mol L-1), subsequently washed with dilute HCl (0.5 mol L-1) and finally rinsed with water. All the solutions were made up by doubly demonized distilled water throughout the work. A stock BDH stock solution of bismuth (1 mg/mL) in dilute nitric acid was used. A series of standard diluted bismuth (III) solutions were then prepared in doubly distilled water by dilution. Stock solutions (0.1% w/v) of the reagent (IId and IV were prepared in DMF). In the cyclic voltammetric experiment, an electrochemical cell composed of three electrodes namely working (Pt wire), counter (Pt wire) and Ag/AgCl reference electrode. The surface area of the counter electrode was 100 times larger than the area of the working electrode. |
| Â |
| Organic synthesis |
| Â |
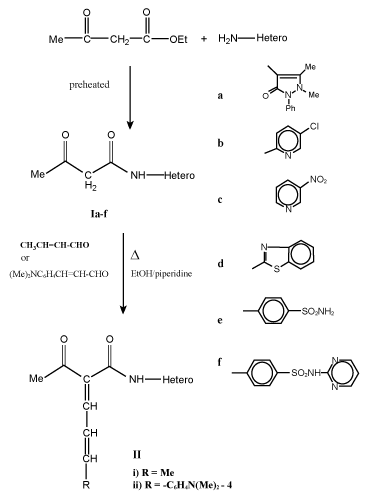
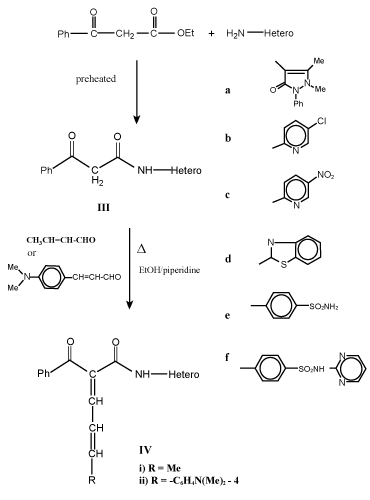
| Preparation of acyl/benzoyl acetanilide derivatives (I and III): To preheated ethyl acetoacetate and/or ethylbenzylacetate (0.01 mol) a selective hetero primary amines and/or sulfa drugs (0.01 mol) were added in dry system then warmed for 10-15 min at 100-110°C, cooled and finally washed with diethyl ether. The resultant solid was dried and crystallized to give I and III respectively (Table 1). |
| Â |
|
|
Table 1: Physical properties of prepared compounds I-IV. |
|
| Â |
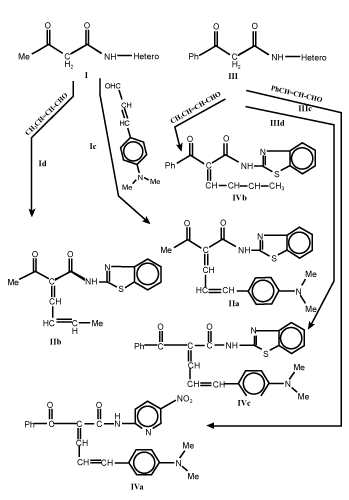
| Formation of 1-acyl/benzoyl-1-anilido-4-methyl/aryl-1, 3-butadiene (II and IV): Equimolar mixture of compounds I and/or III and unsaturated aldehydes as crotonaldehyde and/or 4-dimethylaminocinnamaldehyde in absolute ethanol (100 mL), piperidine (0.5 mL) was heated under reflux for 8 h, cooled. The solvent was removed and the obtained solid was crystallized to give II and IV respectively (Table 1). |
| Â |
| Preparation of the reagent immobilized polyurethane foam packed column |
| Â |
| Polyurethane foam (PUFs) cubes immobilized with the reagent 1-acyl/benzoyl-1-anilido-4-methyl/aryl-1,3-butadiene were prepared by mixing the dried foam cubes with water containing the required weight of the reagent (0.05% w/v) with efficient stirring for 10 min. The reagent foam cubes were then dried to remove the excess solution of the reagent with filter papers as reported earlier [34,35]. The reagent immobilized PUFs were packed separately in the glass columns by applying the vacuum method of foams packing. |
| Â |
| Pre concentration and/or separation of bismuth (III) |
| Â |
| An accurate weight (0.1 ± 0.01 g) of the reagent 1-acyl/benzoyl-1-anilido-4-methyl/aryl-1, 3-butadiene loaded foam cubes was equilibrated with 25 mL of an aqueous solution containing bismuth (III) ions at concentration of 10 μg mL-1. The solutions were then adjusted to the required pH with Britton-Robinson buffer (pH 2.2-11.4). The test solutions were then shaken for two hours on a mechanical shaker. The aqueous phase was then separated out by decantation and the amount of bismuth (III) remained in the aqueous phase was determined with atomic absorption spectrometry at trace concentration at the optimum wavelength. The amount of bismuth (III) retained on the PUFs cubes was determined from the difference between the concentration of bismuth (III) solution before (Co) and after (Ca) shaking with the foam cubes measurements. |
| Â |
| Results and Discussion |
| Â |
| Spectroscopic characterization |
| Â |
| Synthesis of 1, 3-dicarbonyl anilido and their unsaturated derivatives is very simple and of general applicability. It gives pure compounds with improved yields. Thus, warming some hetero primary amines such as 4-aminoantipyrine, 2-amino-5-chloropyridine, 2-amino-5-nitropyridine, 2-amiobenzthiazole and some sulfa drugs as sulfanilamide and sulfadiazine with preheated ethyl acetoacetate and/ or ethyl/benzoyl acetate at 100-110°C for 10-15 min in dry condition led to the formation of acyl/bezoyl acetanilide derivatives I and III (Scheme 1, 2). |
| Â |
|
|
Scheme 1: The formation of acyl/bezoyl acetanilide derivatives I and III. |
|
| Â |
|
|
Scheme 2: The formation of acyl/bezoyl acetanilide derivatives I and III. |
|
| Â |
|
|
Scheme 3: Knoevenagel condensation of compounds I and III. |
|
| Â |
|
|
Scheme 4: A series of open-chain and cycilc structures. |
|
| Â |
| Knoevenagel condensation of compounds I and III with unsaturated aldehydes such as crotonaldehyde and/or 4-dimethylcinnamaldehyde in boiling ethanol with few drops of piperidine as catalyst afforded 1-acyl/benzoyl-1-anilido-4-methyl-1,3-butadiene (II) and 1-acyl/ benzoyl-1-anilido-4-(4`-dimethylaminophenyl)-1,3-butadiene (IV) respectively (Scheme 1-3). Both carbonyl compounds I-IV having a second carbonyl at β-position, are termed as β-diketones. In general hydrogen bonding is possible only in syn form and not in anti form, were the orientation of enolization is towards the aryl or phenyl groups [5] (easily soluble in aq. NaOH). This explains the very high enolic content of 4-aryl-1, 3-diketones and absent in the case of 4-methyl-1, 3-diketones (Scheme 4). UV – Visible spectra of compounds II and IV have two strong bands around 360 and 260 nm which characteristics bands of carbonyl chromophore and the conjugated C=C of butadiene (n-π* and π-π* transition) while that of compounds I and III recorded a low bands at 330 and 240 nm due to a bathochromic shift, indicating the involvement of the two carbonyl groups isolated by methylene group, assigned to the intramolecular charge transfer interaction involving the whole molecule [6]. |
| |
| Â |
| IR spectra of compounds I and III show no characteristic C=O absorption band (at 1725-1710 cm-1) which is present in the spectrum of acetylacetone ~ 1680 cm-1 while that of II and IV recorded of strong absorption bands at 1650 cm-1 of true C=O group. Also, intermolecular as well as intermolecular hydrogen bonding are observed in the regions 2700-2500 and 3000-2900 cm-1 respectively. The presence of absorption bands at 1610-1480 cm-1 (C=C) and 960-900 cm-1 confirm the presence of trans –CH=CH- moiety of compounds II and IV respectively. These vibrations indicate that the transformation of the electronic effect is quite apparent through the part comprising the NH, OH and CO groups. |
| Â |
| 1HNMR spectra of compounds I and III showed a one proton signal at ~15 ppm which confirm the presence of strong intramolecular hydrogen bonded enol proton, in addition, a signal appeared at δ 6.5-6.8 (methine) 6.9-7.9 ppm (aryl) protons. In such systems, the maximum enolization is two especially if containing aryl moieties. On the other hand, 1HNMR spectra of compounds II and IV, showed a resonance signals at 8.0-8.5 ppm (olefinic), 2.5 ppm (methyl protons (due to an allylic coupling between HC=CH and methyl group), in addition of aromatic protons at 6.7-7.7 ppm. Also, the tautomeric forms of compounds I and III is evidence by PMR which showed one resonated singlet for proton linked to sp3-carbon at 4.66 ppm. |
| Â |
| Mass spectroscopy of compounds I-IV give another indication of their stability which shown the degree between ketonic and enolic equilibrium. Also, fragmentation results give us a different ways between Me-CO and Ar-CO structures. A good physico chemical evidence for the presence of enolic and or ketonic tautomers of compounds I and III was deduced from free solubility of their Ar-NHCO derivatives in aqueous NaOH which confirm that the enolization forms take place towards aryl and or phenyl groups [5]. |
| Â |
| Moreover, the proposed structures were also confirmed with the aid of 13C (ppm) NMR of some selected compounds e.g. I d and III d as follows: |
| Â |
| According to the 1H and 13C NMR data, the acy/benzoyl anilide derivatives have mainly an enolic structure with intra molecular hydrogen bond (in solution state), while the styryl of acyl/benzoyl anilides exist predominantly in the cis- form established by some type of interaction. |
| Â |
| Biocidal and analytical applications |
| Â |
| Biocidal effect: Some synthesized compounds were tested in vitro using the agar diffusion disk method [20,21]. The antimicrobial potentialities of the test compounds were estimated by placing pre sterilized filter paper disks (11 mm diameter) impregnated with 50 mg/disk using DMSO as solvent, which showed no inhibition zone (IZ) of tested compounds (mm) were measured after 24 h incubation at 37°C for bacteria and after 5-days incubation at 28°C for fungi. Photochemical probe effects of these compounds can be determined using UV–360 as second test. Compounds III e, d, f and IV e, d, f showed a highly biocidal effect and only compound III e, d, f characterized by photochemical probe action than other prepared compounds. |
| Â |
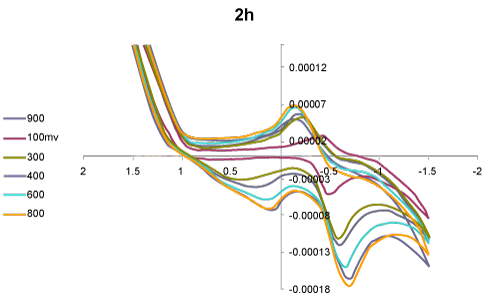
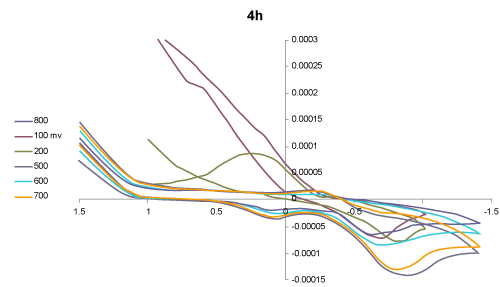
| Voltammetric behaviour: The cyclic voltammograms (CVs) of the two selected compounds 1-acyl-1-anilido-4-methyl-1,3- butadiene, IIa and 1-benzoyl-1-anilido-4- methyl-1,3- butadiene, IV a in DMF -TMA+.Cl- at Pt working electrode versus Ag/AgCl reference electrode at various scan rate were critically investigated. Representative results are shown in figures 1 and 2. The CV of compound IIa (Figure 1) at 100 mV/s revealed two well-defined cathodic peaks in at 0.1 and - 0.75 V versus Ag/AgCl reference electrode. On the reverse scan, one well defined anodic peak at -0.15 V suggesting the irreversible nature of the observed electrochemical process of the compound in the employed potential window (-2.0-2.0 V). Moreover, on raising the scan rate (>100 mV s-1), the potential of the two cathodic peaks were shifted cathodically, while the anodic peak shifted anodically confirming the irreversible nature of the observed electrochemical processes [37]. The observed cathodic peaks are most likely assigned to the reduction of the carbonyl group via 2H+/2e in two successive one electron / one proton reduction steps [38]. Continuous scan of the CV significantly decreased the peak current height indicating passivity of the surface of the Pt electrode via formation of polymeric oxidation product or fouling of the Pt electrode by the produced reduction products suggesting prior adsorption on the surface of the electrode in the potential range [38]. |
| Â |
|
|
Figure 1: Cyclic voltammograms of the compound 1-acyl-1-anilido-4- methyl-1, 3- butadiene, IId in DMF-TMA+.Cl- at Pt working electrode vs. Ag/AgCl electrode at various scan rates. |
|
| Â |
|
|
Figure 2: Cyclic voltammograms of 1-benzoyl-1-anilido-4- methyl-1, 3- butadiene,
IVd in DMF-TMA+.Cl- at Pt working electrode vs. Ag/AgCl electrode at various scan rates. |
|
| Â |
| The CV of compound IV a in DMF- TMA+.Cl- at the Pt working electrode versus Ag/AgCl reference electrode at various scan rates (Figure 2) showed two reduction peaks at 0.1-0.15 and -0.65-0.8 V coupled with one broad anodic peak in the potential range 0.3-0.4 V at 50-1000 mVs-1 scan rate versus Ag/AgCl reference electrode. The observed cathodic peaks are safely assigned to the reduction of the carbonyl group in two successive redox steps involving H+/e [38]. The peak –peak potential difference between the cathodic and anodic peaks (Ep,c – Ep,a) indicated that, the observed redox processes are irreversible. On raising the scan rate both cathodic and anodic peaks are shifted to more negative and positive potential, respectively confirming the irreversible nature of the observed redox process [38]. The plot of the cathodic peak current (ip, c) versus the square root of the scan rate was linear confirming that the electrochemical processes is diffusion controlled processes [38]. The plot of Ep,c versus log scan rate was found linear at Ag/AgCl. Thus, it can be concluded that, the first reduction processes of the compound precede according to the well known electrode- coupled (EC) chemical reaction mechanism [37,38]. Therefore, the product of this reduction step undergoes a very rapid follow-up chemical reaction. |
| Â |
| Retention behaviour of bismuth (III) onto reagent 1-acyl/benzoyl- 1-anilido-4-methyl/aryl-1, 3-butadiene treated tolyurethane foam: Preliminary batch experiments employing polyurethane foams immobilized with the selected reagent 1-acyl/benzoyl-1-anilido-4- methyl/aryl-1, 3-butadiene abbreviated AMB for the extraction of bismuth (III) from aqueous solution was critically examined. The data showed that, reasonable amount of bismuth (III) ions are retained onto the reagent immobilized polyurethane foams from the aqueous solution. Thus, the effects of various parameters that control the retention of bismuth (III) ions from the aqueous solutions have been carried out in the subsequent work. |
| Â |
| The amount of bismuth (III) ions extracted from the aqueous solutions by polyurethane immobilized reagent AMB was found to depend on the solution pH. Thus, the sorption profiles of cadmium (II) at reasonable concentration (10.0 μg mL-1) from the test aqueous solution (100 mL) containing excess of KCl, KBr or KI at different pH (pH 3-10) employing Britton – Robinson buffer onto the reagent loaded foams were examined. After shaking the solutions for two hours, the amount of bismuth (III) remained in the aqueous solution was determined spectrophotometrically at 420 nm as bismuth (III) tetra iodo complex. The amount of bismuth (III) retained, the extraction percentage, %E and the distribution ratio, D was then calculated employing the following equations. The results revealed that, the maximum retention of bismuth (III) by the immobilized reagent PUFs was achieved from the aqueous media containing iodide ions and the sequence of the uptake followed the following order: |
| Â |
| Iodide media> Bromide media > chloride media |
| Â |
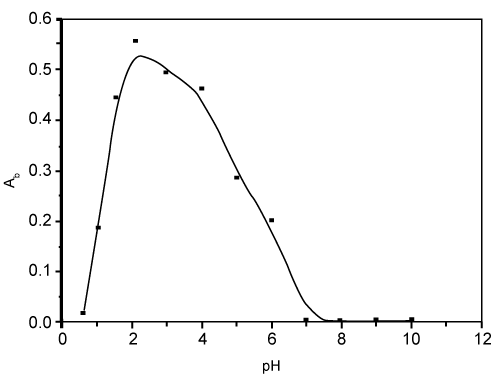
| The results also revealed that, maximum uptake of bismuth (III) from the iodide media was achieved round pH 3 and decreases on increasing the solution pH as shown in figure 3. |
| Â |
|
|
Figure 3: Effect of pH on the sorption of bismuth (III) from the aqueous media containing iodide ions by the reagent AMP- treated PUFs. |
|
| Â |
| At pH 3-3.5, the uptake of bismuth (III) onto the reagent AMP- immobilized polyurethane foams is most likely attributed to the formation of the anionic complex species [BiI4]-2 in the aqueous phase and subsequent formation of the ternary ion associate involving the formation [AMP+.(BiI4)-] or formation of Bi- AMP complex via ligand replacement reaction (or ligand addition mechanism) and formation of high molecular weight complex which in turn retained on the PUFs cubes. Moreover, the possible protonation of the chelating sites either ether and/or urethane linkages in the PUFs may account for the observed behavior. These suggest that the bismuth (II) uptake is most likely preceded by a "solvent extraction" and/or weak base anion exchange mechanism. Similar trend has been observed for the retention of cadmium (II) and other bismuth (III) complex species by methyl isobutyl ketone, diethyl ether, isopropyl ether and PUFs [32-34]. |
| Â |
| The observed decrease on the bismuth (III) retention at pH>3.5 is most likely attributed to the instability of bismuth–iodide or the ternary complex of bismuth – I- AMP due to the hydrolysis of the species formed between [BiI4and the reagent immobilized polyurethane foams. Similar trends were also reported earlier ]- [31]. Thus, a possible "weak base anion ion exchanger" and a “solvent extraction†mechanism of the [BiI4]-(aq) could be proposed for the retention onto the protonated ether (–CH2–HO+–CH2–) oxygen or urethane (–NH+2COO-) nitrogen linkages of the immobilized AMP-PUFs. |
| Â |
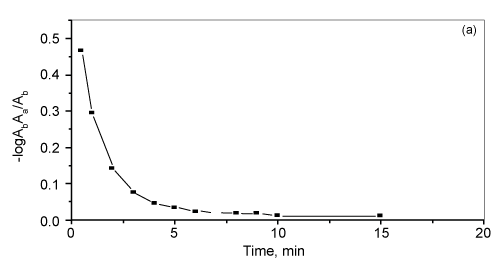
| The effect of contact time and shaking time on the retention of bismuth by the treated AMP- PUFs of bismuth (III) from the aqueous solution containing high excess of KI (5-7% w/v) by AMP-loaded foam was carried out at pH 3.0. The bismuth (II) uptake was fast and reached maximum within ~ 10-15 min contact time. The half-life time (t1/2) of the equilibrium sorption of bismuth (III) as calculated from the plot of -log (Ab-Aa)/Ab versus time onto the reagent immobilized PUFs from the aqueous media to reach 50% saturation of the sorption capacity was found in the range 1-1.5 min (Figure 4). The sorption of bismuth (III) ions was fast within the first 10 min and slightly increased up to a constant value less than 60 min shaking time of both sorbents towards bismuth (III) retention. Thus, a shaking time of 60 min was adopted in subsequent experiments. |
| Â |
|
|
Figure 4: Rate of sorption of bismuth (III) onto the reagent AMP-loaded foams at 35 ºC. |
|
| Â |
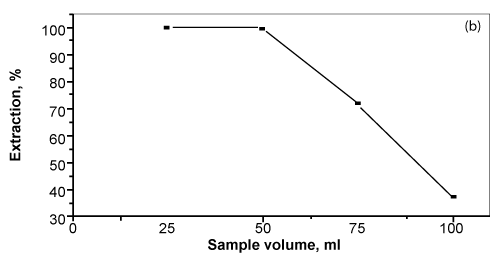
| The influence of sample volume (25-125 mL) of the aqueous solution containing bismuth (III) at pH~2 on the sorption of bismuth (III) by the unloaded PUFS and TZ+.Cl- or ST immobilized PUFs was investigated. The results indicated that, the extraction of bismuth (III) from the aqueous solution containing excess of KI decreases gradually on increasing the sample volume. The results are summarized in figure 5. The uptake of bismuth (III) decreased up to 55% extraction on raising the sample volume from 25 to 125 mL. |
| Â |
|
|
Figure 5: Influence of sample volume (mL) on the sorption percentage of bismuth
(III) onto the reagent AMP immobilized foams. |
|
| Â |
| Conclusion |
| Â |
| The synthesis of novel 1-acyl/benzoyl-1-anilido-4-methyl- butadiene derivatives as chelating agents for the minimization and separation of trace and ultra trace metal ions in drinking and wastewater samples represents great challenges in recent years. Therefore, the synthesis of this class of chemicals and voltammetric behavior of some selected compounds were achieved. The redox behavior of the tested compounds suggest the possible application of the compounds as chelating agent for the determination of ultra trace concentration of heavy metal ions employing differential pulse – adsorptive cathodic stripping voltammetry. Moreover, immobilization of one of the prepared compounds on polyurethane foam solid sorbent as trapping agent for minimization and / or separation of bismuth (III) from industrial wastewater was achieved. However, work is still continuing for application of the compound in cathodic stripping voltammetric procedures for trace metal analysis in different matrices including industrial wastewater. |
| Â |
| Acknowledgement |
| Â |
| The authors would like to thank the Deanship of Scientific Research, King Abdul Aziz University, Saudi Arabia for the financial support under the grant number 428/173 and the Department of Chemistry for the facilities provided in terms the use of the available equipments, chemicals, technicians and other laboratory facilities. One of the authors (Prof R.M. Abdel-Rahman) would like to thank Prof. Z. El-Bazza and his research group, pharmamicrobiological laboratory, National Center for Radiation and Technology, Cairo, Egypt, for carrying out the antimicrobial assays and photo-synthetization activity of cancer. |
| Â |
| |
| References |
| Â |
- Funk RL, Fitzgerald JF, Olmstead TA, Para KS, Wos JA (1993) Titanium-mediated cyclizations of .beta.-keto esters with acetals: a convenient route to 2-carbalkoxycycloalkenones. J Am Chem Soc 115: 8849-8850.
- Zheng H, Swager TM (1994) Octahedral metallomesogens: liquid crystallinity in low aspect ratio materials. J Am Chem Soc 116: 761-762.
- Sievers RE, Turnipseed SB, Huang L, Lagalante AF (1993) Volatile Barium ß-Diketonates for Use as MOCVD Precursors. Coordination Chemistry Reviews 128: 285-291.
- Knoevenagel E, Arnot A (1904) Condensation of salicylaldehyde and cyanoester, benzoylester and acetylacetone. Ber 37: 4496-4499.
- Kailash D (1991) Trans Met Chem 16: 73.
- Abdul Samath S, Raman M, Raman N, Jeayasubramanian K, Ramalingam SK (1992) Trans Met Chem 173.
- Issa YM, Hegazy WH (2000) Preparation and Characterization of some substituted 3-Benzylidine-2, 4- Pentanediones and some of their Metal Complexes. Synth React Inorg Metal–Org Chem 30: 1731-1746.
- Wang QL, Yudao M, Bojun Z (1997) Knoevenagel condensation Catalyzed by USY Zeolite. Synth Commun 27: 4107.
- Veeraraj A, Sami P, Rahman N (2000) Copper(II) complex of 3-cinnamalideneacetylacetone: Synthesis and characterisation. Proc Indian Acad Sci (Chem Sci) 112: Â 515-512.
- Abdel-Rahman R.M (1998) Indian J Chem 27 B 548.
- Abdel- Rahman R.M, Abu-ElWafa , Z. El-Gendy SM (1990) Egyptian J Chem 33: 387.
- Abdel- Rahman RM, M. Seada, M.M. Fawzy (1991) Pak. J. Sci. Ind. Res 34: 465.
- Ramadan AAT, Abdel-Rahman RM, El-Behairy MA, Ismail AI, Mahmoud MM (1993) The thermodynamics of complexation of transition and lanthanide ions by ethylaminobenzylidenehydrazino)-5,6-diphenyl-1,2,4 triazine (HIPHT). Thermochimica Acta 222: 291-303.
- Ramadan A.A.T, Abdel- Rahman R.M, Seada M (1992) Asian J Chem 4: 186.
- Arrieta, F. Dietze, G. Mann, Beyer L, Hartung H (1988) J Prak Chem 330: 111.
- Govindarajan VS (1980) Turmeric--chemistry, technology, and quality. Crit Rev Food Sci Nutr 12: 199-301.
- P.T. Tuchinda (1994) J Indian Chem Soc 71: 509.
- Venugopalan P, Krishnankutty K (1998) Metal Chelates of Curcuminoids. Synth React Inorg Metal- Org Chem 28: 1313-1325.
- Cerchiaro G, Ferreira AM, Teixeira AB, Magalhaes HM, Cunha AC, et al. (2006) Synthesis and crystal structure of 2,4-dihydro-4-[(5-hydroxy-3-methyl-1-phenyl-1H-pyrazol-4-yl)imino]-5-methyl-2-phenyl-3h Pyrazol- 3-one and its copper(II) complex. Polyhedron 25: 2055-2064.
- Â Krishnankutty K, Ummathur MB (2006) J Indian Chem Soc 83: 639-663.
- Venugopalan P, Krishnankutty K (2001) J Indian Chem Soc 78: 472.
- Venugopalan P, Krishnankutty K (2003) J Indian Chem Soc 68: 334.
- Bashammakh AS, Al-Shareef FM, El-Shahawi S (2008) Anal Sci.
- Saad EM, Mansour RA, El-Asmy A, M.S. El-Shahawi, Talanta, 2008, in press.
- El-Shahawi MS, Almehdi MA (1995) J Chromatog A 697: 185.
- El-Shahawi MS, Nassif HA (2003) Retention and thermodynamic characteristics of mercury(II) complexes onto polyurethane foams. Analytica Chimica Acta 481: 29-39
- Abou-Mesalam MM, El-Naggar IM, Abdel-Hai MS, El-Shahawi MS (2003) Use of open-cell resilient polyurethane foam loaded with crown ether for the preconcentration of uranium from aqueous solutions. J Radioanal Nucl Chem 258: 619- 625.
- El-Shahawi MS, El-Sonbati MA (2005) Retention profile, kinetics and sequential determination of selenium(IV) and (VI) employing 4,4'-dichlorodithizone immobilized-polyurethane foams. Talanta 67: 806-815.
- Bashmakah AS, Bahaafi SO, El- Shahawi MS (2007) Talanta, 73: 297.
- El-Shahawi MS, Othman MA, Abdel-Fadeel MA (2005) Kinetics, thermodynamic and chromatographic behaviour of the uranyl ions sorption from aqueous thiocyanate media onto polyurethane foams. Analytica Chimica Acta 546: 221-228.
- El-Shahawi MS, Al-Mehrezi RS (1997) Detection and semiquantitative determination of bismuth(III) in water on immobilized and plasticized polyurethane foams with some chromogenic reagents. Talanta 44: 483-489.
- El-Wakil AM, El-Shahawi MS, Farag AB (1990) Trace Analysis by Direct Spectrophotometric Measurements on Polyurethane Foam. Determination of Chromium (VI) as Blue Perchromic Acid with Unloaded and Tri-n-Butylphosphate Loaded Foam. Anal Lett 23: 703-717.
- El-Shahawi MS, Aldhaheri SM (1996) Preconcentration and separation of acaricides by polyether based polyurethane foam. Analytica Chimica Acta 320: 277-287.
- Farag AB, Soliman MH, Abdel-Rasoul OS, El-Shahawi MS (2007) Sorption characteristics and chromatographic separation of gold (I and III) from silver and base metal ions using polyurethane foams. Analytica Chimica Acta 601: 218- 229.
- El-Shahawi MS, Hassan SSM, Othman AM, El-Sonbati MA (2008) Retention profile and subsequent chemical speciation of chromium (III) and (VI)) in industrial wastewater samples employing some onium cations loaded polyurethane foams. Microchem J 89: 13-19.
- Zaki MTM, Abdel-Rahman RM, El-Syed AY (1995) Use of arylidenerhodanines for the determination of Cu(II), Hg(II) and CN- . Analytica Chimica Acta 307: 127-138.
- Manjaoui A, Haladjian J, Bianco P (1990) Electrochemical properties of pyrolytic graphite electrodes modified through adsorbed proteins. Electrochimica Acta 35: 177-185.
- Bard AJ, Faulkner LR "Electrochemical Methods, Fundamentals and Applications", Wiley, New York 1980, P.218.
|
| Â |
| Â |