Sphenoid Aspergilloma: Diagnosed as a Malignancy: A Case Report
Received: 17-Jan-2015 / Accepted Date: 05-Feb-2015 / Published Date: 11-Feb-2015 DOI: 10.4172/2161-119X.1000190
Abstract
Purpose: This case study was undertaken to demonstrate the important aspects of the differentiation between a fungal infections of the sphenoid sinus vs a diagnosis of cancer. It is important to consider fungal disease in the differential diagnosis when treating masses in the sinuses. A 55 year old female employee was exposed to a waterdamaged office that had fungal and bacterial growth. She developed a sphenoid mass that was first diagnosed as cancer. After surgery, radiation, chemotherapy and a second biopsy she discharged fungal hyphae from the opened sphenoid sinus.
Methods: In 2005 her workplace was noted to have water intrusion and was inspected and tested for the presence of fungi and Gram negative bacteria. Wipe samples of dust were collected for culturing and identification of mold and bacteria. Biopsy specimens were tested by PCR DNA analysis for species of mold. The biopsy specimens were reviewed by a Medical Mycologist. The histology slides were stained with Giemsa. Sphenoid discharged materials were stained with fungalase.
Results: The sphenoid mass was shown to be an aspergilloma, Aspergillus terreus. Mycotoxins detected in urine were macrocyclic trichothecenes, aflatoxins and ochratoxin. The sphenoid aspergilloma completely resolved following oral and intranasal administration of antifungals. Multiple organ symptoms resulting from her exposure and chronic inflammation abated following detoxification and supportive antioxidant therapy. Clinical observations and diagnostic testing ruled out other causes, revealing chronic inflammation and an infection resulting from exposure to fungi and bacteria in the work environment.
Conclusions: Sphenoid aspergilloma can be medically treated with a combination of voriconazole and cyclosporine when they are administered intranasally. The required duration antifungal therapy can be determined by DNA PCR in combination with MRI and appropriate follow up. The findings are discussed and the rational for accepting the aspergilloma rather than a sphenoid malignancy is presented. It is imperative that fungal origins be considered in cases of suspected sinus neoplasms.
Introduction
Fungal Rhinosinusitis (FRS) is a relatively common, often misdiagnosed disease process of the paranasal sinuses [1-3]. The incidence of the disease is 37 million cases that encompass a wide spectrum of immune and pathological responses, including invasive, chronic granulomatous and allergic conditions. A recent attempt was made to classify the various types of fungal sinusitis [4]. The current schema still includes 1) invasive diseases (acute invasive, granulomatous invasive and chronic) FRS and 2) noninvasive disease (saprophytic fungal infections, fungal ball and fungus related eosinophilic FRS that includes AFRS (allergic fungal rhinosinusitis). Thus, FRS results from multiple of fungal genera, including Aspergillus species [1-8]. Aspergillus species are involved in invasive FRS in immunocompetent individuals [9-12]. In all cases the condition is refractory to antibiotic regimens and is improved with intranasal antifungals [2,13,14]. The use of corticosteroids should be limited because of the potential for suppression of the neutrophil migration and killing action on fungal spores and hyphae by both neutrophils and macrophages [15-17]. In addition, Aspergillus sinusitis can mimic malignant disease and even appear as pituitary tumor [18-20]. Presented herein is a case of a 55 year old woman who developed a sphenoid sinus infection by Aspergillus terreus. The infection was initially diagnosed and treated as a neuroblastoma and a pituitary adenoma. She had endoscopic surgery followed with radiation and chemotherapy based upon misdiagnosis. Of interest, A. terreus produces mycotoxins citreoviridin, citrinin, and territrems [21]. The bio-complexity of water-damaged indoor environments has been reviewed by two independent sources [22,23].
Methods and Results
The building
The patient worked as a book keeper in an old building that had water intrusion, musty odors, dead animals in the attic and visible mold growth. The building was inspected, tested for mold and partially remediated. Technicians removed bat and bird dropping, nesting material and insulation from the attic area in August, 2005. However visible mold and musty odors were still present. The testing and identification of mold in the office was conducted by Richard L.Lipsey. Ph.D. and Associates, Jacksonville, FL. Swab samples of dust and visible growth were obtained in July 2009. They were sent under chain of custody to EMLab P and K, Cherry Hill, NJ for culturing and identification of molds (MEA medium) and bacteria (TSA medium), wall cavity contamination and ERMI-36. The results are summarized in Table 1. The results showed typical mold and bacteria present that have been identified in water damaged buildings [24-29]. The potential pathogens found in the Gram negative rods are the following genera: Acinetobacter, Klebsiella, Aeromonas, Campylobacter, Pseudomonas, and Legionella. However, bacterial cultures for infections were not performed in this case.
Nonviable spores present in the two wall check samples of Aspergillus/Penicillium Stachybotrys and Cladosporium. MEA cultures from various areas revealed several species of mold, except Aspergillus terreus. Similarly, the Q-PCR (ERMI Tests) did not reveal A. terreus. However, the data did demonstrate the presence of Aspergillus fumigatus, penicillioides, niger, unguis and ustus along with Eurotium amstelodami, three species of Penicillium and Wallemia. Almost all are known producers of mycotoxins of which several have been identified in water-damaged buildings [23-29]. In addition, it is also recognized that analysis or air and bulk samples may not identify all molds and bacterial toxins present in an indoor environment [27-29]. Thus, absence of A. terreus in the limited sampling conducted in the office of this case is not surprising.
The patient
The past medical history of this 55 year old female included: childhood illnesses (measles, mumps, ear infections and Chicken pox). Adult conditions: surgical excision of let lower leg of melanoma with no recurrence, smokes 10 cigarettes per day, hypertension, and hyperlipidemia. No history of asthma or atopic dermatitis. She had a 15 year history of mild rhino-conjunctivitis using OTC self-medication to control symptoms. She had an episode of pneumonia in 1986, successfully treated. At this time tests for TB and Valley Fever were negative. Shortly after beginning employment she began experiencing persistent health conditions that included but were not limited to the following: Headaches, nasal congestion, chronic sinusitis, tearing of eyes, fullness of ears, sneezing, chronic fatigue, decreased sense of smell, and decreased vision of the left eye. Her treating physician sent her to an allergist to determine the nature of her condition (see below).
Following the allergy workup, she continued to have severe health problems that included but not limited to the following: series of upper respiratory infections, continued decline of vision in the left eye, headaches, and congestion from sinusitis. The loss of vision precipitated a cardiovascular work up for stroke, which was negative.
Allergy testing and medications
Prick/Puncture and intradermal testing for inhalant allergens revealed positive reactions to tree pollens; dust mites and mold groups (A. fumigatus, Fusarium, Penicillium, minor reactions to weed pollens, cat, dog, feather and cockroach allergens and negative to grass, and ragweed. Her medications were Nasonex (2 puffs per nostril per day), Prednisone (40 mg/day for a 4 week tapering), Prevacid, Crestor, Premarin, Atenolol, Triamterene/HCTZ, Ecotrin, folic acid, CoQ10, omega-3 fish oil and flaxseed oil. Her condition continued to deteriorate with eventual loss of vision. She was then subjected to a series of MRIs, PET and CT scans to determine the nature of her condition (CT and PET scans and MRIs below).
Initial MRI, CT, PET scans, endoscope, diagnoses and treatment
The initial PET scan performed in January, 2006 was negative for involvement of other areas of the body (data not shown). The MRI scans revealed the following: A skull base tumor that occupied the bilateral sphenoid sinuses extending into the clivus and sella, encroaching into the posterior maxillary sinuses. In addition, involvement of the left lenticular nucleus, left basal ganglia and cerebellum was noted. The tumor had an axial measurement 3.3×4.2 cm and 8.7 cm in the cranial-caudal length. Erosion of the bone included temporal, posterior maxillary sinuses with abutment of the carotid canals bilaterally.
Endoscopic examination revealed a peduncle with a bulbous mass extending from the sphenoid Ostia into the Nasopharynx. The peduncle and mass were surgically removed and sent for pathological examination. The initial diagnosis was esthesionneuroblastoma. However, additional pathology reports did not agree, naming it a neuroblastoma and/or non-secretory adeno-pituitary tumor (see initial pathology below). She was given two courses of Cisplatin Etoposide chemotherapy in October and November, 2005 with minimal tumor response (reduction in size of 20-25%). In January of 2006 she underwent a sphenoid surgery to remove the mass; this also failed to alleviate the condition. Following the surgery and opening of the sphenoid sinuses, she began and continued to discharge material from her nasal cavity. In February and March of 2006 she was given 36 radiation treatments for the suspected malignancy, again with minimal tumor response. At this time fungal sinusitis, which had been rejected by the pathologists, was suspected. The lack of response to radiation and chemotherapy it was decided to seek assistance for possible samples fungal involvement. Nasal discharge material, tissue samples and pathology slides were sent to Dumanov, Mycological Institute for further evaluation (see mycology below). This material was identified by fungalase staining to contain hyphal fragments
| Sample Location | Culture Medium | Fungi CFU/Swab | Culture Medium | BacteriaCFU/Swab |
|---|---|---|---|---|
| Vent–Top | MEA | 840,000 | TSA | 3,000,000 |
| Vent - Bottom | MEA | 1,100,000 | TSA | 60,000 |
| AHU – Bottom Front | MEA | 310,000 | TSA | 110,000 |
| AHU- Bottom Center | MEA | 490,000 | TSA | 3,100,000 |
| AHU- Bottom Back | MEA | 130,000 | TSA | 100,000 |
| AHU – Floor on Left | MEA | 2,500,000 | TSA | 3,00,000 |
| Blackened Paper – Floor | MEA | 1,200,000 | TSA | 31,000,000 |
AHU = Air Handling Unit
Most Common Fungi: Aspergillus niger, Aureobasidium pullulans, Mucor plumbeus, Rhizopus stolonifer, Rhodotorula mucilagenous, Cladosporium, Penicillium spp, Trichoderma hazarianumm, Cunningham elegans, yeast.
Most Common Bacteria: Bacillus spp, Gram negative rods, Gram positive cocci.
Non-Viable Spores: Non-viable spore samples were taken from a wall cavity with a telephone jack: Penicillium/Aspergillus; Cladosporium and Stachybotrys chartarum.
Q-PCR: ERMI tests: ERMI tests were performed on two samples. Identified fungi were: following: Acremonium strictum, Aspergillus fumigatus, penicillioides, niger, unguis, and ustus, Aureobasidium pullulans, Cladosporium herbarium, Eurotium amstelodami, Mucor/Rhizopus, Paecilomyces variotii, Penicillium chrysogenum, purpurogenum, spinulosum and variable, Rhizopus stolonifer and Wallemia sebi
Wall Cavities: Wall check samples at two outlets (electrical and phone jack) collected by Air-0-Cell cassettes (14 L/min for 5 minutes) detected Aspergillus/ Penicillium at 95 and 87.5 % of the total spore counts of 390 and 1,600, respectively. Stachybotrys spores were detected in the phone jack outlet at 0.008 % if the total spore counts.
Table 1: This table summarizes the Fungi and bacteria identified in the building from cultures of swab samples obtained from patient’s office. All areas were contaminated with fungi and bacteria.
Initial pathology
The results of the pathology of the original peduncle and bulbous mass are summarized in Table 2. The original diagnosis was esthesioneuroblastoma based upon positive immuno-histochemical staining for synaptophysin, cytokeratin 903, chromogranin and inconclusive GMC staining. The potential cross-reactivity with of synaptophysin with Leukophysin, chromogranins and other hematopoietic markers were not considered in the final diagnosis [20,30-34]. In addition, fungi are known to disrupt, utilize and disrupt the actin-cytoskeleton matrix during infection [33-36]. Additional biopsies and histological staining were not done to determine if fungal elements were present. The pathology description on the same biopsy specimen also varied from one laboratory to another, resulting in diagnoses of Esthesionneuroblastoma neuroblastoma, olfactory neuroblastoma and pituitary adenoma
MRI
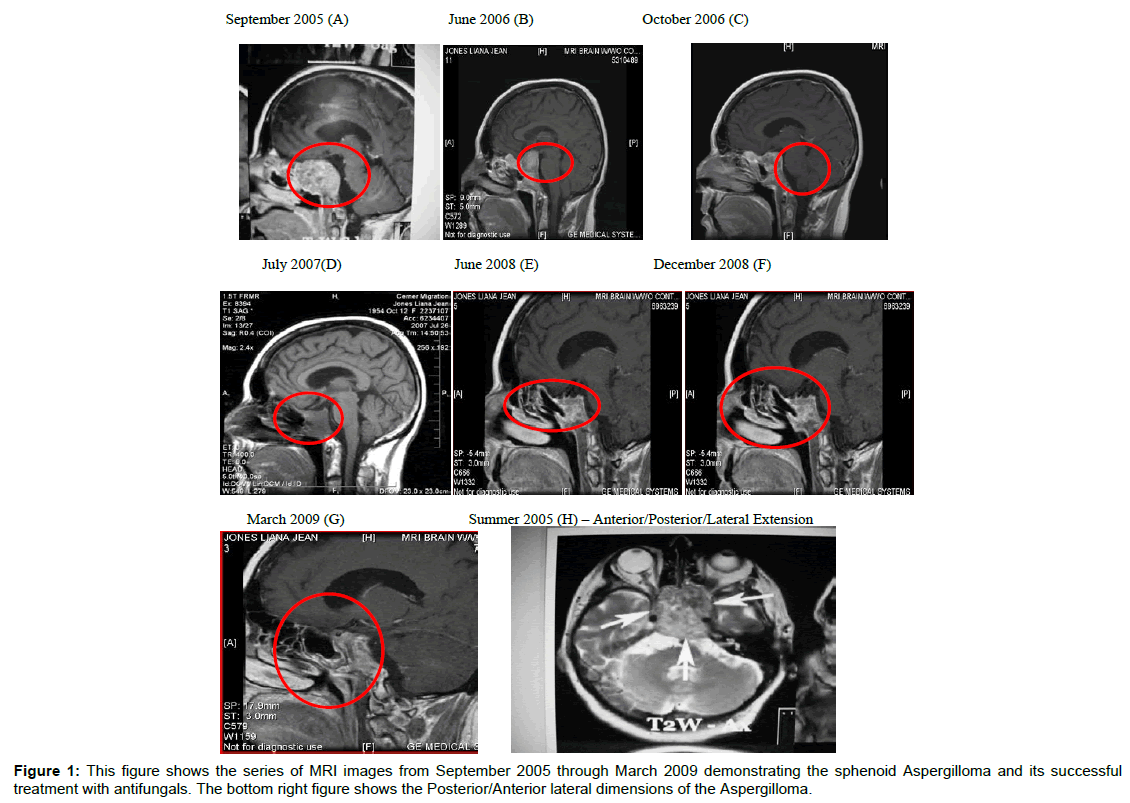
The results of MRI scans are summarized in Table 3 and Figure 1. The MRIs and other scans (data not shown) revealed chronic inflammatory changes in the sphenoid, ethmoid and maxillary sinuses, nasal and paranasal sinus inflammation and chronic inflammatory changes in the left mastoid air cells. In addition, the optic chiasm was displaced by the mass in the sella, along with thinning of adjacent bony cortex. In addition, areas of enhancement were observed bilaterally in the cerebral white matter, the left putamen encephalomalacia changes involving the right caudate nucleus. These observations were apparent even after surgical removal the sphenoid mass (see MRIs, 02/08/07 and 07/26/07). Figure 1 summarizes the results of the MRIs from initial diagnosis if 09/20/05 through 03/30/09. Following radiation treatment (32 in 2006) chemo-therapy (cisplatin/etoposide and surgery the mass was still present in the sphenoid sinuses (Figure 1A-1E). The mass cleared following intranasal antifungal treatments (Figure 1F-1H and Table 4).
| Column1 | Column2 | Column3 | Column4 | Column5 | Column6 | Column7 | Column8 | Column9 |
|---|---|---|---|---|---|---|---|---|
| Place | FMH | UMMC | UMMC3 | Hopkins | AFIP | Pitts | RTL | |
| Date | 10/10/2005 | 1/3/2006 | Aug 30,2006 | 28-Jul-06 | 11/11/2008 | 5/1/2009 | ||
| SX Number | 05-sp-9599 | 01-SC-05-00439 | 01-S-06-00041 Nasal Neoplasm | PHS08-37373 | ||||
| DX | FavorOFN# | NENeoplasm* | Esrhesioneuroblastoma | Pitutary Adenoma | Pit.Adenoma A | NE Neoplasm | ||
| PV Pseudo ros | perivascular pseudorosettes | |||||||
| IPX | ||||||||
| synaptophysin | POS | POS | POS | POS | POS | WK+ | ||
| NSE | POS | POS | POS | |||||
| CD56 | POS | |||||||
| Cam 5.2 | POS | |||||||
| CD99 | NEG | |||||||
| Cytokeratin 903 | POS | NEG | ||||||
| S-100 | NEG | NEG | NEG | NEG | NEG | |||
| HMB-45 | NEG | |||||||
| prolactin | NEG | |||||||
| GH | SCATTERED + | NEG | WK+ | POS | NEG | NEG | ||
| LH | NEG | NEG | ||||||
| FSH | NEG | NEG | ||||||
| TSH | MINIMAL | NEG | ||||||
| ACTH | NEG | POS | NEG | |||||
| Chromagranin | POS | POS | NEG | NEG | NEG | |||
| Keratin | POS | |||||||
| Melan A | NEG | NEG | ||||||
| Silver Stain | NEG | |||||||
| AE1: AE3 | NEG | NEG | NEG | |||||
| EMA | NEG | |||||||
| GMS | NEG | |||||||
| #Olfactory | can't exclude | ˆinconsistent | Difficult see | Agree with | ||||
| Neuroblast | a non-secreting | with OlfNeuro | dx form | Pitts. | ||||
| cannot r/O | pituitary adenoma | |||||||
| pit adenoma |
Table 2: Summary of the Initial Pathology: This table summarizes the 7 different pathology reports done on the original biopsy taken from the nasopharyngeal area described as a peduncle with a bulbous growth arriving from the sphenoid Ostia. Note that the diagnoses were esthesioneuroblastoma, NE neoplasm, and pituitary adenoma.
| MRI Date | Summary of MRI Findings |
|---|---|
| 09/20/05 (A) | Near completeopacification of the sphenoid sinuses with associated thinning of adjacent bony cortex. Destructive changes from either sinusitis or soft tissue mass. |
| 06 2006 (B) | This MRI and the one in figure (C) show minimal response of the sphenoid mass to radiation and chemotherapy. |
| 10/26/06 (C) | large mass replacing the clivus and filling the sella extending into the cavernous sinuses. The mass abuts the undersurface of the optic chiasm. Diffuse inflammatory and post-surgical changes involving the nasal cavity and paranasal sinuses. Diffuse small high signal intensity lesions scattered throughout the cerebral white matter bilaterally, unchanged from prior studies. |
| June/July, 2007 (D) | Post esthesionneuroblastoma. Persistent mass lesion within the clivus. Appears much more cystic. Near optic chiasm with mild mass effect against the chiasm. Left sphenoid more involved than right. Mild maxillary sinus disease. |
| 02/08/07 | There continues to be a large mass replacing the clivus, filling the sella, extending into both cavernous sinuses. Mucosal thickening throughout the sphenoid sinuses bilaterally. Also mild chronic appearing inflammatory changes in the left mastoid. Other paranasalsinuses and right mastoid sinuscells are clear. Very mild diffuse white matter disease, which appears stable when compared to the previousstudy. Multiple new areas of chronic lacuna infarction within the right corona radiate, head of right caudate nucleus,anterior limb of the right internal capsule and right lentiform nucleus (MRI not shown) |
| 07/26/07 (D) | The mass in the clivus and sella decreased to 2×3.4×2.9 from 3.7×2.8×4.1 cm. Mild mucosal thickening in the sphenoid and ethmoid sinuses that has not resolved.Appearance of small cystic area in the right head of caudate nucleus |
| 02/15/08 | Small region of encephalomalacia changes involving the right caudate nucleus. Moderate mucosal thickening of left maxillary sinus as well as ethmoid air cells. Minimal mucosal thickening of sphenoid sinuses. Increased severity of the left maxillary sinus. 8 mm enhancing nodule in the putamen (MRI not shown). |
| 06/30/08 | Pituitary follow-up. Abnormal signal involving the clivus with diffuse enhancement.Pituitary bland measures 1.8×0.9×2.1 cm witheterogeneous enhancement.Abnormal enhancement in the left putamen, 6.4×5.2 mm, stable since previous exam. There are no abnormal signals seen within the brain. – DR. Gray. |
| 09/10/08 (G) | Dr Gray: Follow up of fungus infection in sinuses. There is mucosal thickening in left maxillary sinus with mucous retention cyst, new since previous exam. Mucosal thickening in sphenoid sinus, stable since last exam. Increased signal in the clivus, stable since last exam. Right caudate nucleus without enhancement. Enhancement in left posterior aspect of the putamen (6.9×5.2 mm). No other abnormal signals in the brain. |
| 03/30/09 | Dr. Gray: Mild encephalomalacia, has been stable. Abnormal signal in clivus and mild enhancement of the in the region of cavernous sinus bilaterally. There are no new sites of disease seen. (MRI not shown). |
Table 3: This table summarizes the findings of the MRIs done on the plaintiff’s sinuses from September 09, 2005 to March 30,2009.
Mycology and mycotoxins
Pathology materials were sent to RealTime Laboratories, Carrollton, Texas for differential staining for fungi and RT-PCR-DNA testing for Aspergillus species. In addition, urine specimens were evaluated for the presence of macrocyclic trichothecenes, aflatoxins and ochratoxin as previously reported [37,38]. The differentially stained microscope slides were sent to Joseph Dumanov, Mycological Institute of the Study of Fungal Mold in Human Habitation, Sparta, for pathology evaluation [39].
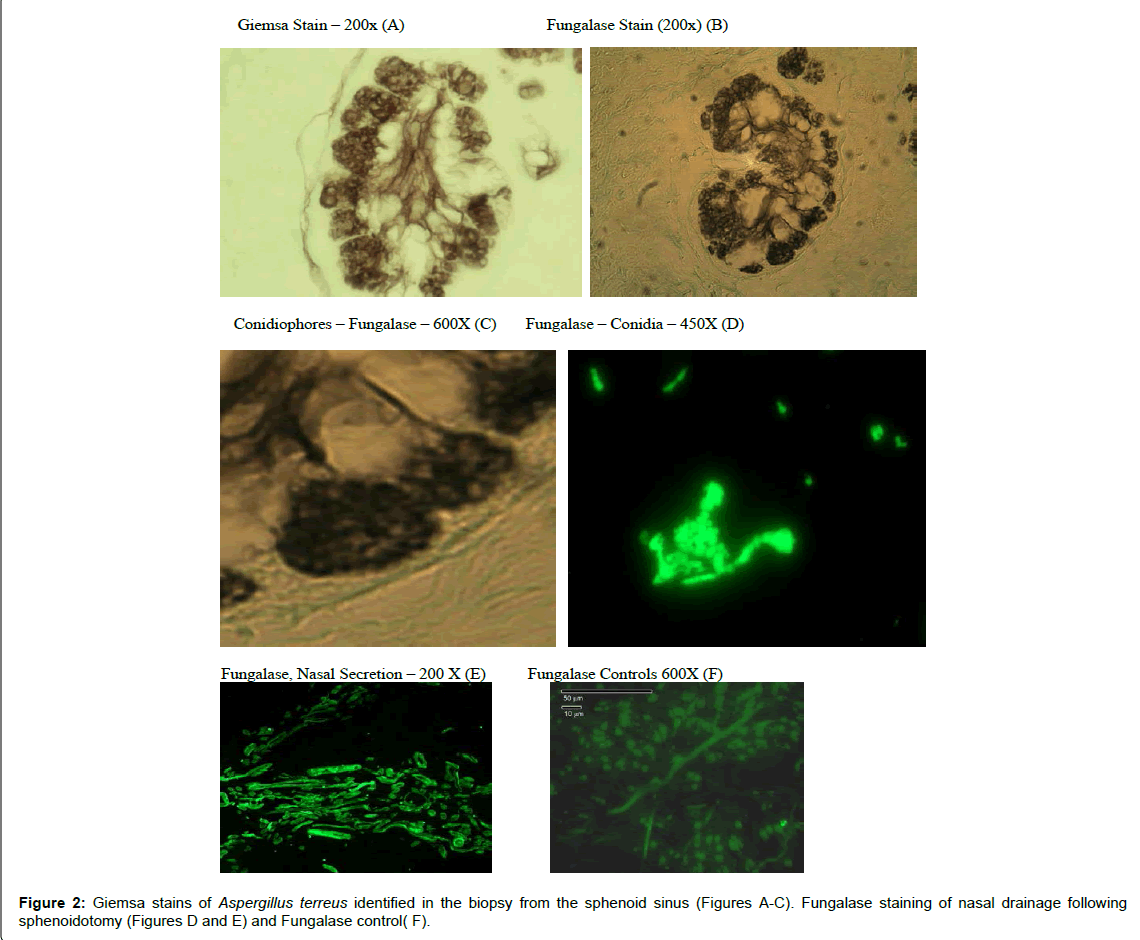
The results of these evaluations were as follows: (1) Fungalase staining revealed septate hyphae and conidia in nasal discharge following sphenoid surgery [39] (Figure 2).
The results of these evaluations were as follows: (1) Fungalase staining revealed septate hyphae and conidia in nasal discharge following sphenoid surgery [39] (Figure 2).
(2) the pathology (Giemsa Stain) evaluation revealed discernable septate hyphae, phialides and conidia characteristic of the genus, Aspergillus (Figure 2).
(3) PCR DNA testing identified Aspergillus terreus in the pathology specimens [39]
(4) The urine was positive for macrocyclic trichothecenes and aflatoxins as listed in Table 5.
Antifungals
The regime of antifungal treatments is summarized in Table 4. Upon Identification of the sphenoid aspergilloma, a regimen of antifungals was initiated. The initial treatment was orally and followed by intranasal sprays. The antifungal regimen eliminated the sphenoid mass. The MRI (March 2009) showed residual sphenoid sinus disease with abnormal signal in the clivus and mild enhancement in the region of the cavernous sinus bilaterally described above. It could be related to mycotic fungus infection versus mucocele. The findings in this area have been stable since previous exam. There were no new sites of disease seen (Figure 1E-G).
| Date | Antifungal | Route – Dose |
|---|---|---|
| 6/3/2007 | Fluconazole | Oral -150 mg/day |
| 14-Jun-07 | Fluconazole Itraconazole | Oral – 150 mg/day Oral – 250 mg/day |
| 10-Jul-07 | Itraconazole Fluconazole | Oral - 200 mg/day x 4 days Oral – 150 mg/day |
| 13-Aug-07 | Fluconazole Fluconazole | Oral – 600 mg/day Oral – 150 mg/day |
| 21-Aug-07 | Ketoconazole Cyclosporine | Intranasal - 4 times/day/per nostril (2%) |
| 9-Sep-07 | Amphotericin + Ketoconazole Nasal Spray | Intranasal – 5 mg/ml Ketoconazole (2%). 2X /day, once per nostril |
| 11-Apr-08 | Voriconazole and Cyclosporine Itraconazole | Intranasal – Dosage Not Available |
Table 4: This table summarizes the administration of antifungals beginning in June 2007 through April 2008.
Discussion
The role of mold in chronic rhinosinusitis was introduced by Ponikau et al., [1]. Since then numerous reports have appeared demonstrating that mold are associated with Fungal Rhinosinusitis (FRS) [1-5,8-14,40]. An attempt to classify the disease led to the following classification of FRS: 1) Invasive disease (acute, granulomatous and chronic); 2) Noninvasive: (saprophytic fungal infestations, fungal ball, and fungus eosinophilic FRS that includes AFRS – Allergic AFR [4]. FRS results from multiple fungal genera, including Aspergillus species [1-8] Aspergillus species are often involved in invasive FRS [10-12]. The invasive FRS requires surgical intervention and favorably responds to oral as well as intranasal antifungals [1-4,11,14,40]. Invasive FRS is associated with erosion of surrounding bone, intracerebral extension and vascular invasion [4,9,11,14]. The use of corticosteroids should be limited because of the potential suppression of neutrophil migration and oxidative burst of neutrophils and macrophages [15-17]. Finally, Aspergillus sinusitis can mimic malignant disease and even appear as a pituitary adenoma [18-20]. With respect to A. terreus it has been identified as the causative fungus in bronchitis, Onchymycosis, pulmonary Aspergillosis, and sphenoid sinusitis with orbitocranial extension [41-45].
The patient presented herein had a 15 year history of mild-rhinoconjunctivitis that was symptomatically treated with OTC medications. Commensurate and shortly after employment in a water-damaged building she developed multiple symptoms that included: headaches, nasal congestion, chronic sinusitis, fullness of ears, sneezing, fatigue, tearing of eyes, decreased sense of smell, and loss of vision in the left eye. Allergy workup demonstrated positive reactions to tree pollens, dust mites, and mold (A. fumigatus, Fusarium, and Penicillium). The symptoms and sinus involvement are consistent with microbial growth in water-damaged buildings and FRS [4,46,47]. However, because of the loss of vision she underwent diagnostic MRIs, CT scan and PET studies that can detect invasive fungal infections and determine the cause of lost vision [48-51]. The results these of diagnostics demonstrated maxillary, ethmoid and mastoid sinuses involvement was well as a large mass in the sphenoid that appeared to be pressing on the optic nerves. The sphenoid mass appeared to cause loss of bony structures in the area (Figure 1). Unfortunately, the initial diagnostics were interpreted as a malignancy treated with chemotherapy, radiation and surgical intervention. None of the cancer treatments were successful in alleviating the mass. Because of the persistence of the mass, nasal discharge and sphenoid tissue samples were examined for evidence of fungal growth. Aspergillus was detected in the biopsy and RT-QPCR identified Aspergillus terreus (Figure 1). In addition, hyphae fragments and conidia were identified in nasal discharges by fungalase staining (Figure 1). As a result oral and intranasal antifungals were initiated in June, 2007. MRIs images from studies in June 2009 through March 2009 showed resolution of the sphenoid mass (Figure 2E-2G). The enhancements seen the MRIs in the caudate nucleus, putamen, white matter, lentiform nucleus and other areas of brain may be indicative of intracerebral extension. The detection of mycotoxins in the urine samples (Table 1) are consistent with previous findings that exposure to molds in water-damaged homes and buildings results in the presence of these toxins in autopsy and biopsy specimens from various organs, sinuses, canine sebaceous tumors and urine [37,40,50,51].
In this case the issue of whether or not the presence of Aspergillus terreus in the sphenoid sinus resulted from the initial diagnosis of a malignancy, rather than being a primary infection that was misdiagnosed. We have several reasons to state that the fungal infection of the sinus was primary [52-54]. These are as follows:
1) At least three different cancers were diagnosed by different pathologist (NE neoplasm, Esthesionneuroblastoma, and Pituitary adenoma (Table 2)
| Date | Aflatoxins (ppb)1 | Trichothecenes (ppb)2 |
|---|---|---|
| 3/27/2007 | 21 | 1.51 |
| 4/11/2007 | 5 | 0.53 |
| 6/3/2007 | 4 | 1.38 |
| 7/10/2007 | 12 | 3.44 |
| 7/14/2007 | 9 | 0.73 |
| 8/21/2007 | 5 | 21.3 |
| 12/12/2007 | 20 | 1.9 |
1,2Limit Detection for Aflatoxins - <1.8 ppb (negative); 1.8-2.0 ppb (equiv0cal); ≥ 2.0 ppb (positive).
Table 5: Urine Mycotoxins. This table summarizes the concentrations of Aflatoxins and Macrocyclic Trichothecenes detected in urine specimens. Mycotoxins continue to be released from intracellular storage and appear in the urine during detoxification.
2)The immunochemistry used markers that are known to cross react with other cellular constituents of normal cells [30-34,48-51] leading to a possible misdiagnosis
(3) The sphenoid tumor did not favorably respond to either radiation or chemotherapy
(4) The opening of the sphenoid sinus lead to the nasal discharge of fungalase positive mycelial fragments (Figure 2).
(5) Treatment with antifungals successfully removed the sphenoid mass without a recurrence of the mass (Figure 1G and 1H)
(6) Fungal infections are difficult to identify histological material; without appropriate staining appropriate staining procedures [54]. In this case Giemsa and fungalase staining identified the fungal hyphae as well as Aspergillus (Figure 2) and finally
(7) Aspergillus species are involved in fungal sinusitis, rhinosinusitis, and pituitary masses [1-4,13-14,17,18].
Conclusion
The patient in this case study was initially misdiagnosed as a malignancy associated with the sphenoid mass. The initial sphenoid sinus biopsy the lesion was diagnosed as an Esthesionneuroblastoma. Subsequent diagnoses included NE neoplasm and pituitary adenoma. The lesion did not respond to surgical biopsies, radiation treatment and chemotherapy. Subsequent follow up for potential fungal involvement demonstrated the presence of Aspergillus terreus DNA in the sphenoid sinus along with fungalase positive hyphal fragments and conidia in nasal discharged fluids. Mycetoma should be included in the differential diagnosis of masses in the sinuses. Finally it is currently recognized that molds and bacteria present in water-damaged indoor environments leads to multiple health problems of the occupants [52-54].
References
- Ponikau JU, Sherris DA, Kern EB, Homburger HA, Frigas E, et al. (1999) The diagnosis and incidence of allergic fungal sinusitis. Mayo ClinProc 74: 877-884.
- Ponikau JU, Sherris DA, Kita H, Kern EB (2002) Intranasal antifungal treatment in 51 patients with chronic rhinosinusitis. J Allergy ClinImmunol 110: 862-866.
- Ponikau JU, Sherris DA, Kephart GM, Kern EB, Congdon DJ, et al. (2005) Striking deposition of toxic eosinophil major basic protein in mucus: implications for chronic rhinosinusitis. J Allergy ClinImmunol 116: 362-369.
- Chakrabarti A, Denning DW, Ferguson BJ, Ponikau J, Buzina W, et al. (2009) Fungal rhinosinusitis: a categorization and definitional schema addressing current controversies. Laryngoscope 119: 1809-1818.
- Braun H, Buzina W, Freudenschuss K, Beham A, Stammberger H (2003) 'Eosinophilic fungal rhinosinusitis': a common disorder in Europe? Laryngoscope 113: 264-269.
- Katzenstein AL, Sale SR, Greenberger PA (1983) Allergic Aspergillus sinusitis: a newly recognized form of sinusitis. J Allergy ClinImmunol 72: 89-93.
- Schubert MS (2009) Allergic fungal sinusitis: pathophysiology, diagnosis and management. Med Mycol 47 Suppl 1: S324-330.
- Shin SH, Ponikau JU, Sherris DA, Congdon D, Frigas E, et al. (2004) Chronicrhinosinusitis: an enhanced immune response to ubiquitous airborne fungi. J Allergy ClinImmunol 114: 1369-1375.
- Reddy CE, Gupta AK, Singh P, Mann SB (2010) Imaging of granulomatous and chronic invasive fungal sinusitis: comparison with allergic fungal sinusitis. Otolaryngol Head Neck Surg 143: 294-300.
- Milroy CM, Blanshard JD, Lucas S, Michaels L (1989) Aspergillosis of the nose and paranasal sinuses. J ClinPathol 42: 123-127.
- Siddiqui AA, Shah AA, Bashir SH (2004) Craniocerebralaspergillosis of sinonasal origin in immunocompetent patients: clinical spectrum and outcome in 25 cases. Neurosurgery 55: 602-611.
- Sivak-Callcott JA, Livesley N, Nugent RA, Rasmussen SL, Saeed P, et al. (2004) Localised invasive sino-orbital aspergillosis: characteristic features. Br J Ophthalmol 88: 681-687.
- Dennis DP (2003) Chronic sinusitis: defective T-cells responding to superantigens, treated by reduction of fungi in the nose and air. Arch Environ Health 58: 433-441.
- Dennis DP, Robertson D, Curtis L, Black J (2009) Fungal exposure endocrinopathy in sinusitis with growth hormone deficiency: Dennis-Robertson syndrome. ToxicolIndust Health 25:669-680.
- Gan WQ, Man SF, Sin DD (2005) Effects of inhaled corticosteroids on sputum cell counts in stable chronic obstructive pulmonary disease: a systematic review and a meta-analysis. BMC Pulm Med 5: 3.
- Palmer LB, Greenberg HE, Schiff MJ (1991) Corticosteroid treatment as a risk factor for invasive aspergillosis in patients with lung disease. Thorax 46: 15-20.
- Philippe B, Ibrahim-Granet O, Prévost MC, Gougerot-Pocidalo MA, Sanchez Perez M, et al. (2003) Killing of Aspergillusfumigatus by alveolar macrophages is mediated by reactive oxidant intermediates. Infect Immun 71: 3034-3042.
- Larranaga J, Fandiño J, Gomez-Bueno J, Rodriguez D, Gonzalez-Carrero J, et al. (1989) Aspergillosis of the sphenoid sinus simulating a pituitary tumor. Neuroradiology 31: 362-363.
- Lee JH, Park YS, Kim KM, Kim KJ, Ahn CH, et al. (2000) Pituitary aspergillosis mimicking pituitary tumor. AJR Am J Roentgenol 175: 1570-1572.
- Daghistani KJ, Jamal TS, Zaher S, Nassif OI (1992) Allergic aspergillus sinusitis with proptosis. J LaryngolOtol 106: 799-803.
- Samson RA, Peterson SW, Frisvad JC, Varga J (2011) New species in Aspergillus section Terrei. Stud Mycol 69: 39-55.
- WHO (2009) Dampness and Mould: Who Guidelines for indoor air quality, Euro non serial publications.
- Thrasher JD, Crawley S (2009) Thebiocontaminants and complexity of damp indoor spaces: more than what meets the eyes. ToxicolInd Health 25: 583-615.
- Thrasher JD, Gray MR, Kilburn KH, Dennis DP, Yu A (2012) A water-damaged home and health of occupants: a case study. J Environ Public Health 2012: 312836.
- Bloom E, Nyman E, Must A, Pehrson C, Larsson L (2009) Molds and mycotoxins in indoor environments--a survey in water-damaged buildings. J Occup Environ Hyg 6: 671-678.
- Polizzi V, Delmulle B, Adams A, Moretti A, et al. (2009) JEM spotlight: Fungi, mycotoxins and microbial volatile organic compounds in mouldy interiors from water-damaged buildings. J Environ Monit 11:1949-58.
- Taubel M, Sulyok M, Vishwanath V, Bloom E, Turunen M, et al. (2011) Co-occurrence of toxic bacterial and fungal secondary metabolites in moisture-damaged indoor environments. Indoor Air 21: 368-375.
- Smoragiewicz W, Cossette B, Boutard A, Krzystyniak K (1993) Trichothecenemycotoxins in the dust of ventilation systems in office buildings. Int Arch Occup Environ Health 65: 113-117.
- Tuomi T, Reijula K, Johnsson T, Hemminki K, Hintikka EL, et al. (2000) Mycotoxins in crude building materials from water-damaged buildings. Appl Environ Microbiol 66: 1899-1904.
- Ebener U, Wehner S, Cinatl J, Gussetis ES, Kornhuber B (1990) Expression of markers shared between human haematopoietic cells and neuroblastoma cells. Anticancer Res 10: 887-890.
- Abdelhaleem MM, Hatskelzon L, Dalal BI, Gerrard JM, Greenberg AH (1991) Leukophysin: a 28-kDa granule membrane protein of leukocytes. J Immunol 147: 3053-3059.
- Abdelhaleem MM, Hameed S, Klassen D, Greenberg AH (1996) Leukophysin: an RNA helicase A-related molecule identified in cytotoxic T cell granules and vesicles. J Immunol 156: 2026-2035.
- Mendes-Giannini MJ, Taylor ML, Bouchara JB, Burger E, Calich VL, et al. (2000) Pathogenesis II: fungal responses to host responses: interaction of host cells with fungi. Med Mycol 38 Suppl 1: 113-123.
- Kogan TV, Jadoun J, Mittelman L, Hirschberg K, Osherov N (2004) Involvement of secreted Aspergillusfumigatus proteases in disruption of the actin fiber cytoskeleton and loss of focal adhesion sites in infected A549 lung pneumocytes. J Infect Dis 189: 1965-1973.
- Taylor MJ, Ponikau JU, Sherris DA, Kern EB, Gaffey TA, et al. (2002) Detection of fungal organisms in eosinophilicmucin using a fluorescein-labeled chitin-specific binding protein. Otolaryngol Head Neck Surg 127: 377-383.
- Hooper DG, Bolton VE, Guilford FT, Straus DC (2009) Mycotoxin detection in human samples from patients exposed to environmental molds. Int J MolSci 10: 1465-1475.
- Hooper DG, Bolton VE, Sutton JS, Guilford T, et al. (2012) Assessment of Aspergillusfumigatus in Guinea pig bronchoalveolar lavages and pulmonary tissues by culture and realtime polymerase chain reaction studies. Int J MolSci 13:726-36.
- Dumanov J (2008) Pathology report by Dr. Dumanov. Mycological Institute for the Study of Fungal Mold in Human Habitations, Sparta, NJ.
- Thrasher JD, Gray MR, Kilburn KH, Dennis DP, Yu A (2012) A water-damaged home and health of occupants: a case study. J Environ Public Health 2012: 312836.
- Chrdle A, Mustakim S, Bright-Thomas RJ, Baxter CG, Felton T, et al. (2012) Aspergillus bronchitis without significant immunocompromise. Ann N Y AcadSci 1272: 73-85.
- Fernandez MS, Rojas FD, Cattana ME, Sosa Mde L, Mangiaterra ML, et al. (2013) Aspergillusterreus complex: an emergent opportunistic agent of Onychomycosis. Mycoses 56: 477-481.
- Guinea J, Padilla C, Escribano P, Muñoz P, Padilla B, et al. (2013) Evaluation of MycAssayâ„¢ Aspergillus for diagnosis of invasive pulmonary aspergillosis in patients without hematological cancer. PLoS One 8: e61545.
- Akhaddar A, Gazzaz M, Albouzidi A, Lmimouni B, Elmostarchid B, et al. (2008) Invasive Aspergillusterreus sinusitis with orbitocranial extension: case report. SurgNeurol 69: 490-495.
- Schubert MS (2009) Allergic fungal sinusitis: pathophysiology, diagnosis and management. Med Mycol 47 Suppl 1: S324-330.
- Takahashi H, Hinohira Y, Hato N, Wakisaka H, Hyodo J, et al. (2011) Clinical features and outcomes of four patients with invasive fungal sinusitis. AurisNasus Larynx 38: 289-294.
- Brewer JH, Thrasher JD, Straus DC, Madison RA, Hooper D (2013) Detection of mycotoxins in patients with chronic fatigue syndrome. Toxins (Basel) 5: 605-617.
- Brewer JH, Thrasher JD, Hooper D (2013) Chronic illness associated with mold and mycotoxins: is naso-sinus fungal biofilm the culprit? Toxins (Basel) 6: 66-80.
- Gray MR, Thrasher JD, Crago R, Madison RA, Arnold L, et al. (2003) Mixed mold mycotoxicosis: immunological changes in humans following exposure in water-damaged buildings. Arch Environ Health 58: 410-420.
- Fisk WJ, Eliseeva EA, Mendell MJ (2010) Association of residential dampness and mold with respiratory tract infections and bronchitis: a meta-analysis. Environ Health 9: 72.
- Alrajhi AA, Enani M, Mahasin Z, Al-Omran K (2001) Chronic invasive aspergillosis of the paranasal sinuses in immunocompetent hosts from Saudi Arabia. Am J Trop Med Hyg 65: 83-86.
- Aribandi M, McCoy VA, Bazan C 3rd (2007) Imaging features of invasive and noninvasive fungal sinusitis: a review. Radiographics 27: 1283-1296.
- Gorovoy IR, Kazanjian M, Kersten RC, Kim HJ, Vagefi MR (2012) Fungal rhinosinusitis and imaging modalities. Saudi J Ophthalmol 26: 419-426.
Citation: Gray MR, Thrasher JD, Hooper D, Dumanov MJ, Cravens R, et al. (2015) Sphenoid Aspergilloma: Diagnosed as a Malignancy: A Case Report. Otolaryngology 5:190. DOI: 10.4172/2161-119X.1000190
Copyright: © 2015 Gray MR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 16485
- [From(publication date): 5-2015 - Nov 21, 2024]
- Breakdown by view type
- HTML page views: 12047
- PDF downloads: 4438