Research Article Open Access
Small changes of hydration and Air Displacement Plethysmography
| Coralie Lauley2, Concepcion Gonzalez2, Caroline Perlemoine2, Henri Gin2 and Vincent Rigalleau1* | |
| 1Nutrition-Diabétologie, USN, Hôpital Haut-Lévêque, avenue de Magellan, 33600 Pessac | |
| 2Université Victor Segalen-Bordeaux 2, 146, rue Léo Saignat, 33000 Bordeaux, France | |
| Corresponding Author : | Vincent Rigalleau Nutrition-Diabétologie, USN Hôpital Haut-Lévêque, avenue de Magellan 33600 Pessac, and Université Victor Segalen- Bordeaux 2 146, rue Léo Saignat, 33000 Bordeaux, France Tel. 33 5 57 65 60 78 Fax: 33 5 57 65 60 79 E-mail: vincent.rigalleau@wanadoo.fr |
| Received November 21, 2011; Accepted January 14, 2012; Published January 19, 2012 | |
| Citation: Lauley C, Gonzalez C, Perlemoine C, Gin H, Rigalleau V (2012) Small changes of hydration and Air Displacement Plethysmography. J Obes Weig los Ther 2:111. doi:10.4172/2165-7904.1000111 | |
| Copyright: © 2012 Lauley C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Obesity & Weight Loss Therapy
Abstract
Background: Do current-life changes of hydration influence the assessment of small (~1kg) body composition changes by Air Displacement Plethysmography (ADP) ?
Methods: In ten normal subjects, ADP measurements were performed before and after the ingestion of 1.055L of water, and then after miction. The analysis were conducted with the subjects carriying external charges of 1.055L of oil (density 0.9), and 1.055L of a fat-free-mimicking solute (density 1.1).
Results: Ingesting and carrying 1.055L of water led to a similar significant increase in fat-free mass (ingested: +0.98±0.78 kg, carried: +1.01±0.98). A significant reduction of the body volume was detected after the miction (0.286±0.152L), without any significant change of body composition. In the three situations (basal-hydrated-after miction), fat and fat-free loads were discriminated, closest to reality in well-hydrated subjects. The measured changes were correlated with the true changes, but the %fat of the changes were underestimated (real: +22±34%, measured: -3±70%; p<0.001), and biased (Bland & Altman procedure): the more the change was fat, the less ADP underestimated its fatness.
Conclusions:ADP discriminates ~1kg alterations in fat vs fat-free mass. The analysis should be performed in similar conditions of good hydration.
| Keywords |
| Air Displacement Plethysmography; Hydration; Fat mass; Fat-free mass |
| Abbreviations |
| ADP: Air Displacement Plethysmography; BIA: Body Impedance Analysis |
| Background |
| The analysis of body composition provides important information on physical status. Numerous physical or isotopic methods are used to distinguish fat and fat-free mass, but only a few, such as Body Impedance Analysis (BIA) [1] or Dual Energy X-ray Absorptiometry (DEXA) [2] are applicable to clinical practice. Body weight changes during lifestyle interventions [3], or the pharmacological treatment of obesity [4], although clinically relevant, are usually limited only to a few kg. Measurement of body composition would help evaluate the beneficial (or deleterious) effects of these small changes if the techniques are accurate. Whether BIA [5] or DEXA [6] are sufficiently accurate for this purpose is questionable. |
| Air Displacement Plethysmography (ADP) is a promising alternative to the traditional body composition techniques [7]. The commercially available device for ADP (BOD POD) is a safe, quick, reliable and valid method for evaluating body composition in a wide range of subject types, agreeing within 1% with the reference hydrostatic weighing technique [8]. By contrast to BIA and DEXA, ADP offers the unique possibility of verification by weighing and then measuring subjects in the ADP chamber with and without loads of known composition. This approach allowed us to demonstrate that ADP could distinguish moderate changes (~2 kg) in fat and fat-free mass in normal subjects [9]. Further work in obese subjects confirmed this accuracy, but also showed that the results could be biased in cases of alterations in hydration [10], a recognized problem in the analysis of body composition [11]. Does the ingestion of water, or a simple miction, modify the ADP results? Does it alter the detection of small (~1 kg) fat (density =0.9) or fat-free (density =1.1) changes? Could the combination with BIA, as used in three compartment models [12], improve the results? |
| In ten normal volunteers, we compared the results of ADP, before and after ingestion of water, and miction. These comparisons were also performed with BIA. The body composition changes (ADP) after drinking water was also compared to the carrying of a similar water load in a closed bottle. The detection of fat and fat-free loads in the three situations (before/after drinking/after miction) were then compared, and we analyzed whether the hydration of the loads influenced the results. |
| Methods |
| Subjects |
| Ten subjects (5 women, 5 men; age 22±1 yrs, BMI: 22.9±2.1) participated to the study. Informed consent was obtained from each subject, and the study was approved by the ethical committee of our institution. |
| Loads |
| Four loads of identical volumes were employed: |
| The subjects carried three closed bottles (1.055 L, measured by water displacement), containing: |
| -water for the water load (weight: 1.006 kg), |
| -sunflower oil for the fat load (weight: 0.949 kg, density: 0.90), |
| -30 g/100 mL glucose solute for the fat-free load (weight: 1.160 kg, density: 1.10). |
| In each case, a few coins were added to the bottle to adjust its weight and obtain the desired density despite the small influence of the plastic of the bottle and its cap. |
| The oral water load (1.055 L, 1.006 kg) was completely ingested by the subjects. |
| Body composition assessment |
| Weight was measured as part of the BOD POD procedure. The BOD POD was calibrated for an empty chamber and a known volume (49.771 L cylinder) before each measurement. The subjects were weighed, and then entered the BOD POD chamber, wearing only underclothes and a swimcap. Duplicate measurements of body volume were performed according to the BOD POD manufacturer’s recommendations (Life Measurement Instruments, California, USA); a third measurement was performed when they differed by more than 150 mL. Predicted lung volume was used for the calculation of body volume, using adult-specific equations [13]. Fat and fat-free mass were calculated using the equation of Siri [14]. |
| The impedances were measured using a BIA system (Analycor, Eugédia, France). Externally applied, current-inducing spot electrodes were positioned on the dorsal side of the right foot and the left hand. Body impedance at low frequency (5 Hz) was used to determine the volume of extracellular water, and measurements at higher frequency (100 kHz) reflected total body water [15]. |
| Study design |
| All the subjects were studied in the fasting state, and underwent 10 ADP measurements in the following order (each measurement took five to ten minutes): |
| 0) Initial analysis (no ingestion, no bottle), |
| 1) No ingestion, water bottle (+1.006 kg Fat-free, 100% hydrated), |
| 2) No ingestion, oil bottle (+0.949 kg Fat, 0% hydrated), |
| 3) No ingestion, glucose bottle (+1.160 kg Fat-free, 73% hydrated), |
| 4) After ingestion, no bottle (+1.006 kg Fat-free, 100% hydrated), |
| 5) After ingestion, oil bottle (+1.006 kg Fat-free, +0.949 kg Fat, 37% hydrated), |
| 6) After ingestion, glucose bottle (+2.166 kg Fat-free, 80% hydrated), |
| 7) After miction, no bottle (+0.735±0.146 kg Fat-free, 100% hydrated), |
| 8) After miction, oil bottle (+0.735±0.146 kg Fat-free, +0.949 kg Fat, 43.2±4.9% hydrated), |
| 9) After miction, glucose bottle (+1.895±0.146 kg Fat-free, 83.4±1.2% hydrated), |
| - as the weight of urine was 0.270±0.146 kg (Volume: 0.286±0.152 L), and the hydration of fat-free mass was assumed to be 73% (11). |
| BIA were performed at steps “0”, “4” and “7”. At step 0, the muscular strength was determined with a hand-grip dynamometer (Takei, France). |
| Statistical analysis |
| Fat and fat-free mass with and without the loads were compared by paired t tests. Measured changes in fat and non-fat mass were compared and correlated (linear regression analysis) to the true changes with a Bland & Altman procedure using the true changes as reference. The relation between the errors (measured less true change of fat and fatfree mass) and the relative hydration of the loads was also tested by linear regression. SPSS 10.1 software was used for the calculations. Results are presented as mean±SD with p<0.05 considered significant. |
| Results |
| Influence of water ingestion and miction on ADP results (Table 1). As shown in the Table 1, the ingestion of 1.055 L of water was correctly detected as an increase in fat-free mass, with no significant effect on the fat mass. The changes in fat and fat-free mass were similarly detected with the ingestion of water (+0.98±0.78 kg fat-free and + 0.02±0.76 kg fat), and the carrying of the water bottle (+1.01±0.98 kg fat-free and + 0.05±0.95 kg fat): in both cases, the fat mass with the water load did not differ from that without, whereas the fat-free mass increased significantly (p<0.005 by ingesting water, p<0.01 by carrying it externally). The miction did not significantly modify the fat and fatfree mass, although its small change (0.286±0.152 L) was detected as fat by ADP. |
| Changes in fat and fat-free mass assessed by ADP with loads of 0.9 and 1.1 densities (Table 2). It can be seen from Table 2 that the fat mass changes were systematically underestimated, and fat-free changes overestimated. On all occasions, the fat load led to a significantly (p<0.05) higher increase in fat mass than did the fat-free load, and the fat-free load led to a significantly higher increase in fat-free mass than did the fat load, with a ~1 kg difference as expected: the two differing loads were systematically discriminated. But the fat changes were less underestimated and fat-free changes less overestimated, after drinking water (before and after the miction). |
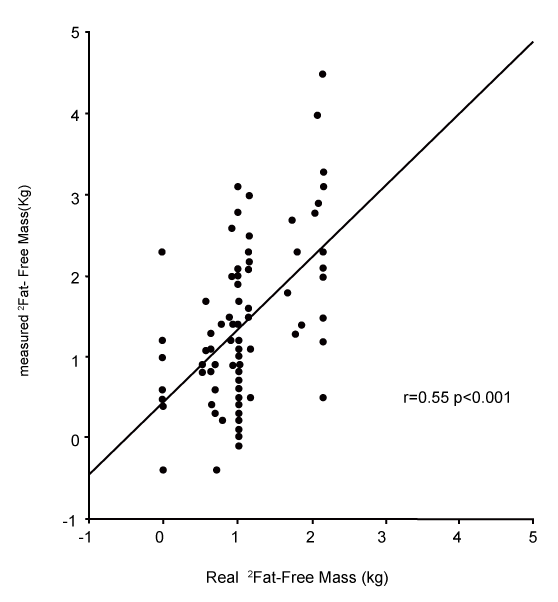
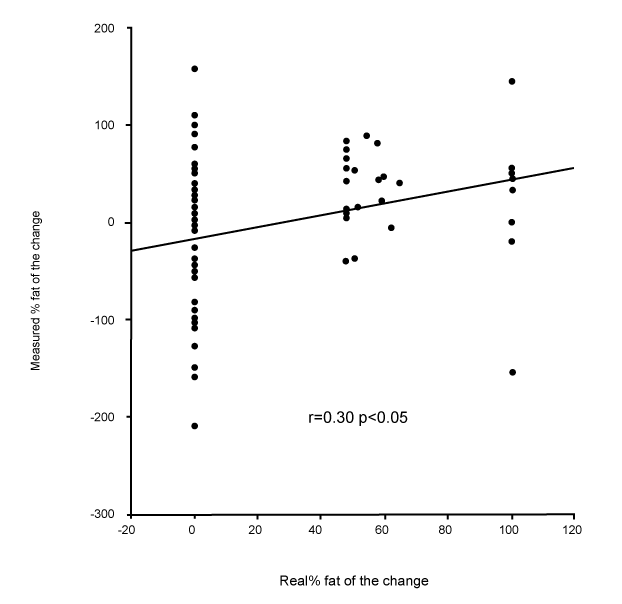
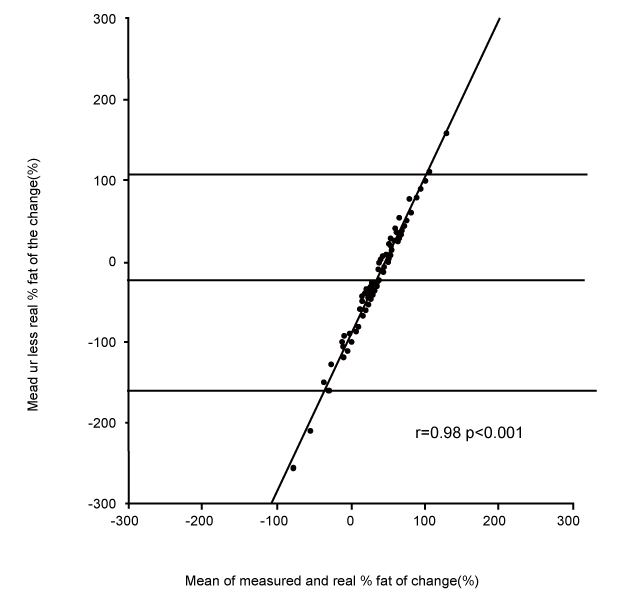
| Changes in fat and fat-free mass assessed by ADP. For each of the ten subjects, the composition of 9 distinct loads were analyzed. For the 90 induced alterations in body composition, the mean true change in fat-free mass was +1.07±0.61 kg, and the ADP overestimated it by +1.40±0.97 kg (p<0.001). This measured change of fat-free mass was significantly correlated with the true change (r=0.55, p<0.001, Figure 1). The mean true change in fat mass was +0.31±0.45 kg, the ADP underestimated it at +0.02±0.86 kg (p<0.001). This measured change in fat mass was significantly correlated with the true change (r=0.41, p<0.001). The mean true %fat of the change was +22.7±34.9%; ADP underestimated it by -3.6±70.8% (p<0.001). This measured %fat change was significantly correlated with the true % (Figure 2A: r=0.30, p<0.05), but according to the Bland & Altman procedure it was biased (Figure 2B: 2SD = 136%, r=0.98, p<0.001): the more the change was composed of fat, the less ADP underestimated its fatness. The % hydration of the loads was correlated with the error (r=0.22, p<0.05), but this contribution was no longer significant after taking account of the bias by the Bland & Altman procedure (p=0.38). |
| Effects of water ingestion and miction on BIA results (Table 3). Due to the large dispersion in the results, no significant changes were detected, except a paradoxical reduction in intracellular water after the ingestion of the water: we considered these results to be aberrant, and did not try to use them to correct the ADP results. |
| Correlation of fat-free mass with muscular strength. ADPmeasured fat-free mass was highly correlated with muscular strength (r= 0.94, p<0.001). Although significant, the correlation between BIAmeasured fat-free mass was weaker (r=0.75, p<0.05). |
| Discussion |
| Our results support the interest of ADP for studying the composition of small body weight changes. The carrying of ~1 kg loads led to significantly different increases in fat (load of 0.9 density) and fat-free mass (load of 1.1 density), as detected by ADP in ten normal subjects. The results would presumably have been the same if the loads had been incorporated to the body of the subjects instead of carried in plastic bottles, as the ingestion of 1.055 L water gave similar results to its addition in an externally beared bottle. ADP therefore discriminates ~1 kg changes in fat from fat-free mass, which improves our previous reports suggesting that ~2 kg changes were discriminated in similar numbers (n=10) of subjects [9,10]. The use of loads of densities calibrated to the postulated densities of fat and fat-free mass instead of just water and oil as previously, probably explains this better accuracy: although water belongs to fat-free mass, its density is lower than 1.1, so it was less distinguished from fat than the 1.1 density load used in the present study. The use of closed instead of open bottles may also have contributed to the better accuracy, as small volumes of trapped air are known to influence the ADP results [16]. Short term changes in hydration did not prevent the discrimination of fat from fat-free loads by ADP: the differences were quite similar before and after miction, and the ingestion of 1L of water was adequately detected as an increase in fat-free mass. But from the practical point of view of an investigator who wants to determine the composition of a few kg alteration in body weight during a longitudinal study, the results in Table 2 indicate that the ADP measurements require similar conditions of hydration for each analysis: a +1 kg fat gain was analyzed as a mainly fat gain after drinking, and mainly fat-free before. The results in the Table 2 also suggest that the best results will be obtained if studying well-hydrated subjects. In such conditions, ADP appears as a quick, safe, clinically usable and reliable tool for studying the composition of small body weight changes. |
| Our study however also shows the limitations of this method. The ADP measured changes of fat and fat-free mass were correlated with the true changes, but they were not exactly the same. For example, the addition of 0.949 kg of fat (step 2) was measured as a +0.22±0.68 kg increase in fat mass (range: -1.4 to +1.3 kg fat): in common with other physiological parameters, individual results should therefore be interpreted with caution. It is of particular note though that the results are biased: changes in fat mass were underestimated, confirming our previous findings in normal subjects [9]. In this earlier study, we failed to detect the overestimation of fat-free changes, that were in fact water changes. Here, the use of a 1.1 density load enabled this discrimination. Because the density of water differs from the density of fat-free mass, hydration status and changes are a source of uncertainty for any twocompartment model analysis of body composition. It should be noted that the ADP-detection of the water load (ingested or carried) as an increase in fat-free mass, which seems satisfactory at first sight, is in fact another reflection of the ADP bias: due to its low density, the water should have been partly detected as fat. However, varying the water loads from 0 to 100% did not modify the ADP bias, and further work will be required to explain this bias. At baseline, the addition of 0.949 kg fat was detected more as a fat-free than a fat mass increase (Table 2). Although this result correctly contrasted with the effect of the 1.1 density load, it shows that the changes in fat and fat-free mass as measured by ADP cannot be regarded as quantitatively accurate. On the other hand, as shown by the Bland & Altman procedure, the ADP bias was especial: the lower the percentage fat in the load, the more its %fat was underestimated, which favors the discrimination of differing body composition changes. |
| Some limitations of the present study should be born in mind. First, we only studied the influence of short-term limited (0 to 100% of a ~1kg change) alterations in hydration. Some pathological alterations may in fact be more marked, and combine a decrease in the protein and mineral body content with an increase in hydration (Kwashiorkor, severe renal failure, edematous cirrhosis). In common with other methods for assessing body composition, the validity and interpretation of ADP in such situations are yet to be established. Second, our study concerned normal subjects, whose density was clearly different from the density of water. In obese subjects, the initial density is much closer to the density of water, so changes in hydration may have different consequences as we have already reported [10]. Third, the disappointing aberrant results of BIA according to changes in hydration are in line with other reports (17: -24.5% lean body mass after a haemodialysis session), and they dramatically contrast with the accuracy of the ADP measurements in similar conditions. The poor correlation between muscular strength and BIA-estimated fat-free mass is another reflect of its inferiority. However, we think that testing other BIA devices should be done before excluding three-compartment models based on ADP and BIA measurements. |
| In summary, loads of water were similarly detected by ADP whether they were external, or incorporated within the subjects. ADP discriminates well a +1 kg fat vs +1 kg fat-free increase, with significant differences on a small sample size (n=10). The changes in hydration occurring in normal life (drinking up to one liter, urinating) do not impair this ability, but they influence the results: for longitudinal studies, the subjects should be analyzed in similar conditions of good hydration (before/after miction does not seem very important). Individual and quantitative results should be interpreted with caution, as they are biased. |
| Authors contributions |
| Coralie Lauley, Concepcion Gonzalez and Caroline Perlemoine enrolled the subjects and performed the body composition analysis, Henri Gin supervised the study and assisted in thye finalization of the manuscript, Vincent Rigalleau conceived the study, performed the statistical analysis and wrote the manuscript. |
| Acknowledgements |
| There is no conflict of interest associated with this study. |
| This work was supported by a grant from the ALFEDIAM, and the Institut de Recherche en Nutrition Humaine en Aquitaine. |
| We would like to thank Dr. S. Jarman for revision of the English manuscript. |
References
- Boulier A, Fricker J, Thomasset AL, Apfelbaum M (1990) Fat-free mass estimation by the two-electrode impedance method. Am J Clin Nutr 52: 581-585.
- Heymsfield SB, Wang J, Heshka S, Kehayias JJ, Pierson RN (1989) Dual-photon absorptiometry: comparison of bone mineral and soft tissue mass measurements in vivo with established methods. Am J Clin Nutr 49: 1283-1289.
- Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H (2001)Finnish Diabetes Prevention study group: Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 344: 1343-1350.
- Phelan S, Wadden TA (2002) Combining behavioral and pharmacological treatments for obesity. Obes Res 10: 560-574.
- Heitmann BL (1994) Impedance: a valid method in assessment of body composition? Eur J clin Nutr 48: 228-240.
- Genton L, Karsegard VL, Zawadynski S, Kyle UG, Pichard C, et al. (2006) Comparison of body weight and composition measured by two different Dual-energy X-ray absorptiometrydevices and three acquisition modes in obese women. Clin Nutr 25: 428-437.
- Fields DA, Goran MI, McCrory MA (2002) Body-composition assessment via air-displacement plethysmography in adults and children: a review. Am J Clin Nutr 75: 453-467.
- Ginde SR, Geliebter A, Rubiano F, Silva AM, Wang J, et al. (2005) Air displacement plethysmography: validation in overweight and obese subjects. Obes Res 13: 1232-1237.
- Secchiutti A, Fagour C, Perlemoine C, Gin H, Durrieu J, et al. (2007) Air displacement plethysmography can detect moderate changes in body composition. Eur J Clin Nutr 61: 25-29.
- Le Carvennec M, Fagour C, Adenis-Lamarre E, Perlemoine C, Gin H, et al. (2007) Body composition of obese subjects by Air Displacement Plethysmography: the influence of hydration. Obesity 15: 78-84.
- Wang Z, Deurenberg P, Wang W, Pietrobelli A, Baumgartner RN, et al. (1999) Hydration of fat-free body mass: review and critique of a classic body-composition constant. Am J Clin Nutr 69: 833-841.
- Sallé A, Ryan M, Guilloteau G, Bouhanick B, Berrut G, et al. (2005) 'Glucose control-related' and non-glucose control-related' effects of insulin on weight gain in newly insulin-treated type 2 diabetic patients. Br J Nutr 94: 931-937.
- Crapo RO, Morris AH, Clayton PD, Nixon CR (1982) Lung volumes in healthy non-smoking adults. Bull Eur Physiopathol Resp 18: 419-425.
- Siri WE (1993) Body composition from fluid spaces and density: analysis of methods.1961. Nutrition 9: 480-491.
- Segal KR, Burastero S, Chun A, Coronel P, Pierson RN Jr, et al. (1991) Estimation of extracellular and total body water by multiple-frequency bioelectrical impedance measurement. Am J Clin Nutr 54: 26-29.
- Fields DA, Hunter GR, Goran MI (2000) Validation of the BOD POD with hydrostatic weighing: Influence of body clothing. Int J Obes Relat Metab Disord 24: 200-205.
- Morais AA, Costa RA, Grilo MG, Bezerra ME, Vieira MM, et al. (1996) Measurement of body composition changes during hemodialysis by bioimpedance analysis. Rev Hosp Clin Fac Med Sao Paulo 51: 121-123.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2a | Figure 2b |
Relevant Topics
- Android Obesity
- Anti Obesity Medication
- Bariatric Surgery
- Best Ways to Lose Weight
- Body Mass Index (BMI)
- Child Obesity Statistics
- Comorbidities of Obesity
- Diabetes and Obesity
- Diabetic Diet
- Diet
- Etiology of Obesity
- Exogenous Obesity
- Fat Burning Foods
- Gastric By-pass Surgery
- Genetics of Obesity
- Global Obesity Statistics
- Gynoid Obesity
- Junk Food and Childhood Obesity
- Obesity
- Obesity and Cancer
- Obesity and Nutrition
- Obesity and Sleep Apnea
- Obesity Complications
- Obesity in Pregnancy
- Obesity in United States
- Visceral Obesity
- Weight Loss
- Weight Loss Clinics
- Weight Loss Supplements
- Weight Management Programs
Recommended Journals
Article Tools
Article Usage
- Total views: 6046
- [From(publication date):
January-2012 - Nov 22, 2024] - Breakdown by view type
- HTML page views : 1699
- PDF downloads : 4347
